DBLG1 self-learning algorithm: easy to use for optimized and personalized T1D management was shared by Diabeloop.com, 2 June 2021.
 From its inception, Diabeloop has been developing innovative solutions to automate and personalize Type 1 diabetes management, with a patient-perspective always in sight. The French company aims to empower people living with T1D while optimizing their user experience to make diabetes management easier and less omnipresent. DBLG1 algorithm requires only a minimal patients’ input to deliver a satisfying, automated and personalized experience.
From its inception, Diabeloop has been developing innovative solutions to automate and personalize Type 1 diabetes management, with a patient-perspective always in sight. The French company aims to empower people living with T1D while optimizing their user experience to make diabetes management easier and less omnipresent. DBLG1 algorithm requires only a minimal patients’ input to deliver a satisfying, automated and personalized experience.
Relieving people with Type 1 diabetes from their heavy mental burden begins at the early stages of the experience with DBLG1. To safely automate and personalize insulin delivery, DBLG1’s state-of-the-art algorithm only requires 4 settings to be entered. At set-up, people equipped with DBLG1 enter their body weight, their total daily insulin dose (TDD), their typical meals (in grams of carbs) and their safety basal rate (for open-loop only). With Diabeloop’s algorithm, there is no need to provide complex computations. Users do not have to figure out their meal ratio, their insulin sensitivity correction factor, nor their insulin action curve.
The 4 initial settings entered in the system by the patient are enough for DBLG1 self-learning algorithm to run, calculate and adjust the insulin dose as often as every five minutes, as needed.
Sounds interesting, doesn’t it?! The Savvy Diabetic and Loop and Learn hope to be featuring Diabeloop on one of our upcoming Loop and Learn T1D Speaker Series event in the fall 2021. Stay tuned in! While this is approved in the EU, it is not US FDA approved. But wouldn’t that be amazing?
Read more: DBLG1 self-learning algorithm
Regulate human pancreatic islets for transplantation as organs, not drugs was reported by Dr. Piotr Witkowski for Healio.com/Endocrinology, 14 June 2021.
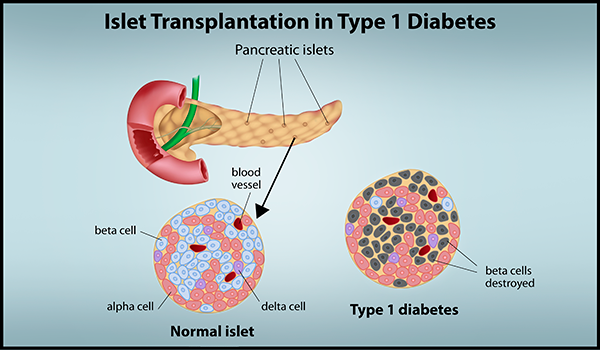 On April 15, an FDA advisory committee concluded that the islet cell therapy donislecel demonstrated a favorable risk-benefit profile for certain patients with difficult-to-control type 1 diabetes. Donislecel (Lantidra, CellTrans) has been shown to lead to insulin independence and decrease the number of severe hypoglycemic events among patients with difficult-to-control type 1 diabetes, based on pooled results of two open-label studies. The therapy comprises islet cells isolated from a deceased donor pancreas. The final cultured cell product is transplanted through infusion in the portal vein going to the liver via percutaneous, laparoscopic or open surgical access. The FDA is expected to render a decision soon as to whether to approve a biologics license application (BLA) for the islets. if this treatment is approved under the current BLA pathway, the consequences for the field of islet transplantation in the U.S. will be detrimental.
On April 15, an FDA advisory committee concluded that the islet cell therapy donislecel demonstrated a favorable risk-benefit profile for certain patients with difficult-to-control type 1 diabetes. Donislecel (Lantidra, CellTrans) has been shown to lead to insulin independence and decrease the number of severe hypoglycemic events among patients with difficult-to-control type 1 diabetes, based on pooled results of two open-label studies. The therapy comprises islet cells isolated from a deceased donor pancreas. The final cultured cell product is transplanted through infusion in the portal vein going to the liver via percutaneous, laparoscopic or open surgical access. The FDA is expected to render a decision soon as to whether to approve a biologics license application (BLA) for the islets. if this treatment is approved under the current BLA pathway, the consequences for the field of islet transplantation in the U.S. will be detrimental.
FDA regulates pancreatic islets as drugs, rather than as human organs. There is plenty of evidence documenting pancreatic islets are not just cells, but rather small organs, and should be regulated as such. In addition, the great variability of pancreas donor factors, islet cellular composition and potency, make it impossible to try to fit islets into the requirements rightly set for advanced cell therapies, or other biologics, where the source material and the final product can be controlled and standardized.
Now, FDA has three options. First, approve the BLA, and in doing so, provide false reassurance that the islet product provided by the commercial BLA-holder is consistently safe and potent. Second, FDA can reject the BLA and continue to require it for anyone desiring to process islets for transplantation outside clinical studies. This will lead to the complete demise of the field and a lack of therapy in use, as medical insurance companies would not pay for unproved islet transplantation. Third — the most rational option — FDA can reject the BLA, acknowledge the scientific evidence of the last 20 years, and allow islets to be regulated as organs for transplantation.
Human islets should instead be regulated under the Health Resources and Services Administration (HRSA) and submitted to the Organ Procurement and Transplantation Network (OPTN) and the United Network for Organ Sharing (UNOS) — all, notably, outside the drug regulations of the FDA. This proposed framework was designed and developed to ensure human organ quality and potency, as well as the safety and effectiveness of transplantation.
Read more: Regulate human pancreatic islets for transplantation as organs
GAD-Alum Immunotherapy: An Interview with Diamyd Medical’s Ulf Hannelius was shared by the Children With Diabetes Editorial Team, 16 June 2021.
 Ulf Hannelius : It is very exciting that we are working on something that is very novel and potentially groundbreaking. The purpose of GAD-Alum is, very much like a vaccine, to specifically re-educate the immune system so that it stops destroying the insulin-producing function. I am also very excited about the fact that, using data from hundreds and hundreds of individuals that have participated in our trials, we have been able to identify a group based on their genetic profile that seems to have a very high likelihood of benefiting from GAD-Alum. This is what is called ‘precision medicine’ and what I believe is the way forward if you want to make a significant impact for any complex disease, including type 1 diabetes.
Ulf Hannelius : It is very exciting that we are working on something that is very novel and potentially groundbreaking. The purpose of GAD-Alum is, very much like a vaccine, to specifically re-educate the immune system so that it stops destroying the insulin-producing function. I am also very excited about the fact that, using data from hundreds and hundreds of individuals that have participated in our trials, we have been able to identify a group based on their genetic profile that seems to have a very high likelihood of benefiting from GAD-Alum. This is what is called ‘precision medicine’ and what I believe is the way forward if you want to make a significant impact for any complex disease, including type 1 diabetes.
The biggest challenge scientifically is that we are dealing with biology and the immune system. As we now know, it is imperative to approach type 1 diabetes as a group of diseases with common symptoms rather than a single, well-defined disease. We are fortunate to have access to a lot of data that has given us the opportunity to take a more data-driven approach as we move into the final development phase of GAD-Alum.
GAD-Alum seems not only to have a high likelihood of clinical effect but also that it is very safe, and the treatment is convenient.
Read more: GAD-Alum Immunotherapy
The FDA Is Failing the American People was written by Vinay Prasad, MD for MedPageToday.com, 16 June 2021. This article is based on the FDA’s recent approval for an Alzheimer’s medication plus several other immunotherapies for cancer which failed to confirm that the drug extended survival or improved quality of life. However, the question is: Is the FDA process flawed or indeed broken?
The case: On June 7, the FDA approve d aducanumab for the treatment of Alzheimer’s disease. The drug received accelerated approval because it showed it could reduce the rate of amyloid plaque on scans. What remains uncertain is whether this reduction in plaque means Alzheimer’s patients live longer or better lives — and notably, the totality of the clinical trial data does not show that. Moreover, the drug has various side effects and a whopping price tag: $56,000 a year.
d aducanumab for the treatment of Alzheimer’s disease. The drug received accelerated approval because it showed it could reduce the rate of amyloid plaque on scans. What remains uncertain is whether this reduction in plaque means Alzheimer’s patients live longer or better lives — and notably, the totality of the clinical trial data does not show that. Moreover, the drug has various side effects and a whopping price tag: $56,000 a year.
Many observers don’t fully appreciate how the FDA has taken a position that is indefensible. The agency does not guarantee that drugs that come to the U.S. market actually help Americans live longer or better lives (beyond what could be achieved without these drugs). At the same time, the FDA insists on interfering in the market and sets arbitrary standards for approval. The combined effect is the worst of all possible worlds: we don’t know if drugs work, and the companies can charge massive prices for them!
The FDA looks like an agency whose goal is to preserve pharmaceutical profits. Coincidentally, among medical reviewers at the agency, the prime destination after working at the FDA is either as a consultant for or employee of a biopharmaceutical company.
Read more: The FDA Is Failing the American People
Most top hospitals charge a more than 5x markup was reported by Tina Reed for Axios.com, 14 June 2021.
Some of the hospitals with the highest revenue in the country also have some of the highest prices, charging an average of 10 times more than the actual cost of the care they deliver, according to new research by Johns Hopkins University provided exclusively to Axios.
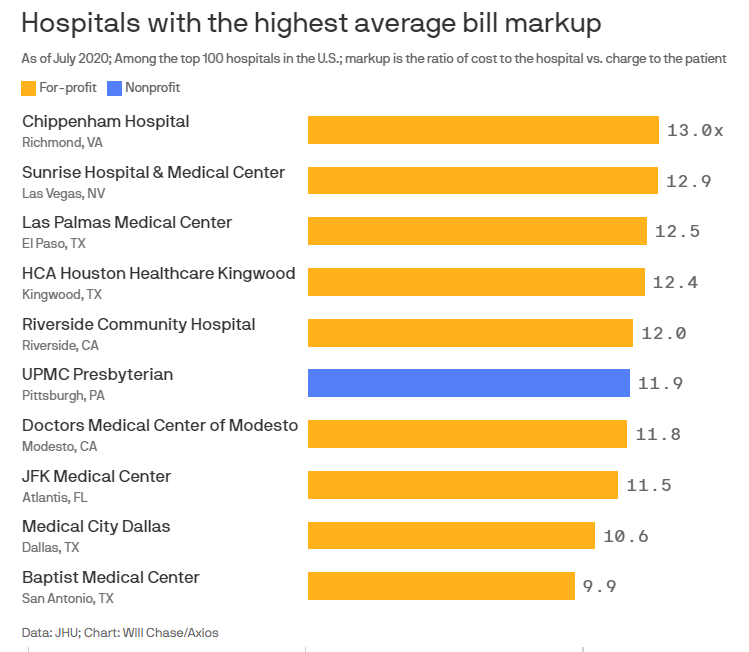
Why it matters: Hospitals each determine their own charges, or list prices. While few patients ever pay those prices, due to negotiated insurance rates, they do affect the uninsured and, experts say, ultimately influence the overall price we all pay.
-
- Of the top 100 largest hospitals in the U.S., 57 were charging more than five times the actual cost, on average, of the care they provided.
- Nine hospitals were charging more than 10 times the actual cost.
- The hospital with the highest average markup was HCA’s Chippenham Hospital in Richmond, with charges at nearly 13 times its cost.
For-profit hospitals had the biggest markups. They made up nine of the 10 hospitals with the biggest markups, and eight of those for-profit hospitals charged more than 10 times their costs.
Read more: Most top hospitals charge a more than 5x markup
Could zinc help control blood pressure? was reported by Robby Berman for MedicalNewsToday.com, 13 June 2021.
 Calcium and potassium are involved in the regulation of blood pressure, but one new study suggests that zinc may also have a part to play. The researchers made this discovery accidentally while they were investigating zinc’s role in rat brain function. If additional research validates the study’s conclusion, the findings could eventually lead to new drugs for controlling hypertension, or high blood pressure.
Calcium and potassium are involved in the regulation of blood pressure, but one new study suggests that zinc may also have a part to play. The researchers made this discovery accidentally while they were investigating zinc’s role in rat brain function. If additional research validates the study’s conclusion, the findings could eventually lead to new drugs for controlling hypertension, or high blood pressure.
“Fundamental discoveries going back more than 60 years have established that the levels of calcium and potassium in the muscle surrounding blood vessels control how they expand and contract,” say the authors of a recent study. However, these scientists unexpectedly found that a metal, zinc, may also play a role in maintaining vascular tone. Senior study author Dr. Scott Ayton, Ph.D., says: “Zinc is an important metal ion in biology and, given that calcium and potassium are famous for controlling blood flow and pressure, it’s surprising that the role of zinc hasn’t previously been appreciated.” Dr. Ayton adds, “Essentially, zinc has the opposite effect to calcium on blood flow and pressure.”
Read more: Could zinc help control blood pressure?
And now for 2 items which are interesting and fun!
Health monitors coming to a loo near you was written by Naama Barak for Israel21c.org, 14 June 2021.
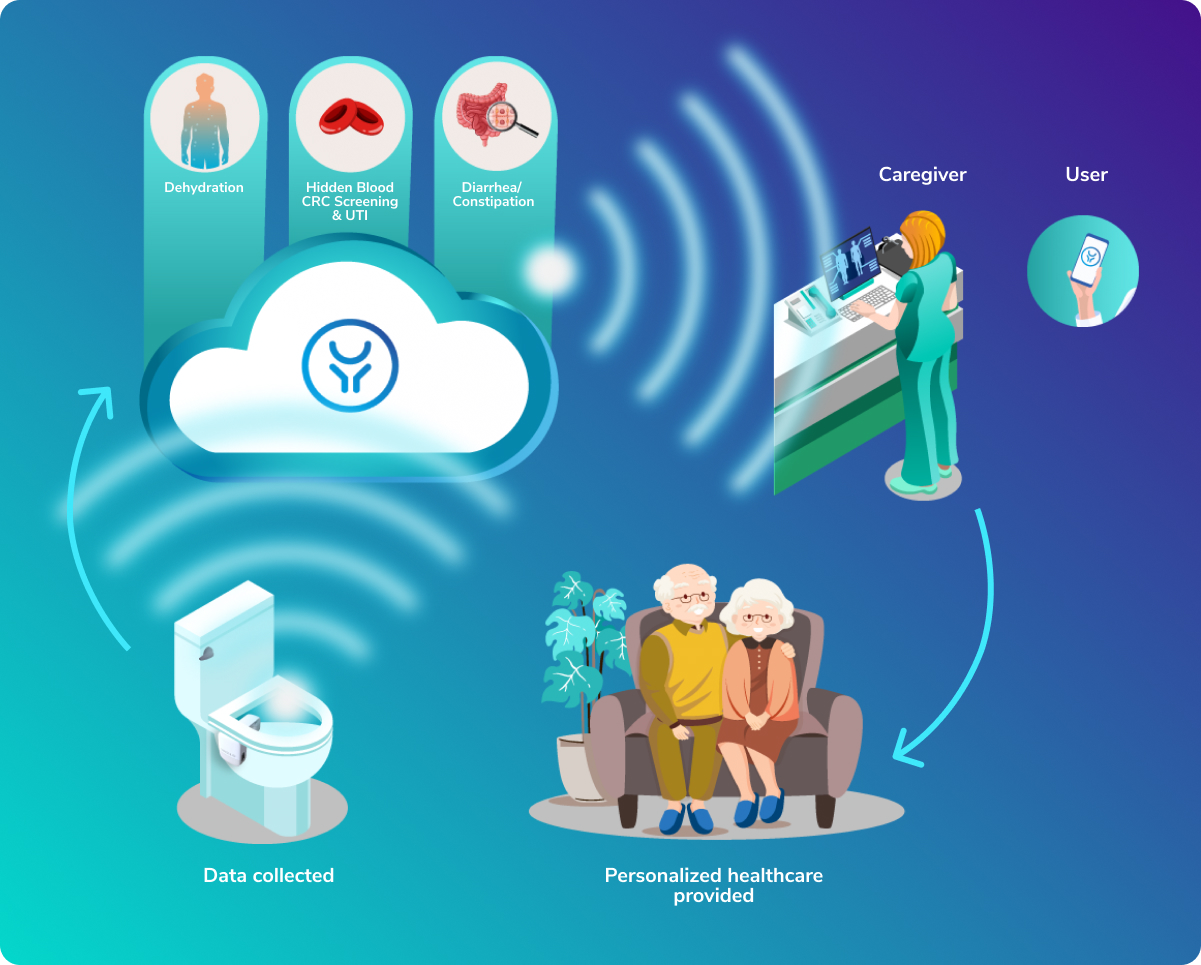 Israeli digital health startup OutSense, whose IoT device detects clinical conditions by analyzing toilet contents, has announced it will begin piloting its technology in the United States later this year. Currently undergoing clinical trials in Israel and a pilot in Japan, OutSense’s optical IoT device attaches to the toilet bowl. It identifies the optical footprint of feces and urine components and sends the data for AI cloud-based analysis before providing indications of various diseases in real-time. The device can identify blood in stool, often a sign of colorectal cancer, and can also diagnose and monitor dehydration, urinary tract infections, diarrhea and constipation. Production and commercial sales are planned for Q1 2022.
Israeli digital health startup OutSense, whose IoT device detects clinical conditions by analyzing toilet contents, has announced it will begin piloting its technology in the United States later this year. Currently undergoing clinical trials in Israel and a pilot in Japan, OutSense’s optical IoT device attaches to the toilet bowl. It identifies the optical footprint of feces and urine components and sends the data for AI cloud-based analysis before providing indications of various diseases in real-time. The device can identify blood in stool, often a sign of colorectal cancer, and can also diagnose and monitor dehydration, urinary tract infections, diarrhea and constipation. Production and commercial sales are planned for Q1 2022.
OutSense CEO Yfat Scialom says, “The ability to partner with a major US healthcare provider serves as a signal to the market that the rules of the game can be changed. It signals that early detection of more diseases through our technology can save huge amounts in healthcare costs, and more importantly save lives.”
Why does this apply to folks with T1D? Well, aside from the detection for cancer, it can also diagnose dehydration (think DKA) and UTI.
Read more: Health monitors coming to a loo near you
Doctor Creates a Straw That Reportedly Cures Hiccups With 92% Success Rate was published by Loukia Papadopoulos for InterestingEngineering.com, 20 June 2021.
 According to the Mayo Clinic, “hiccups are involuntary contractions of the diaphragm — the muscle that separates your chest from your abdomen and plays an important role in breathing. Each contraction is followed by a sudden closure of your vocal cords, which produces the characteristic “hic” sound.”
According to the Mayo Clinic, “hiccups are involuntary contractions of the diaphragm — the muscle that separates your chest from your abdomen and plays an important role in breathing. Each contraction is followed by a sudden closure of your vocal cords, which produces the characteristic “hic” sound.”
BUT … when you have the hiccups you want an instantaneous cure!
Now, scientists have conceived of a straw that takes your hiccups away with one gulp.
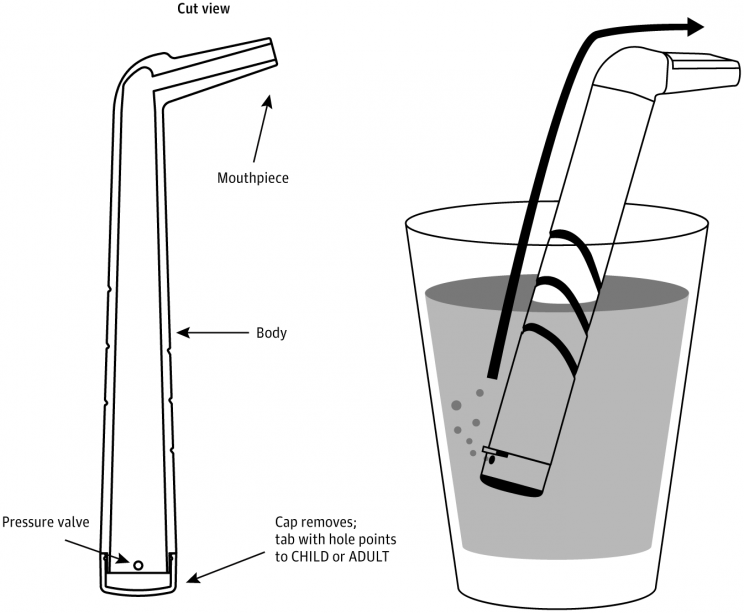
It’s called “the forced inspiratory suction and swallow tool” (FISST) and is patented as HiccAway for $14, designed by Dr. Ali Seifi, Director of the Neuroscience Intensive Care Unit at the University of Texas. It’s quite simple really. It’s a plastic device in an L-shape that has a mouthpiece at one end and an adjustable cap with a pressure valve at the other. Those afflicted by hiccups place the device into a glass of water, take a sip and watch their hiccups disappear.
How does it work? It activates the two nerves responsible for the hiccups. The vigorous suction required to draw water up through the device activates the phrenic nerve to trigger a contraction of the diaphragm. Meanwhile, the swallow activates the vagus nerve. Keeping these two nerves busy negates them the chance to trigger a hiccup.
It’s quite ingenious really and in a study published in the Jama Network it was found to work in 92% of cases. It should be noted however that the study did not have a control group and relied on self-reported results.
Read more: Straw That Reportedly Cures Hiccups With 92% Success Rate


Well the new loop pump sounds interesting. But i think the real idea here is retraining the immune system. I wonder would that not have some potentially powerful problems?
I mean consider retraining the immune system to no longer see Beta cells as the enemy? Would that induce Celiac? Cancer? RA? All of this is spooky. I think leaving the immune system be and modifying the beta cells to with stand the attack sounds much easier.
The autoimmune system is so complex, I think we should accept it like it is and concentrate on better islets.
As for testing my poop ? Yeah that sounds like fun. Not.
rick