Australian scientists develop pain-free blood sugar test for diabetics was reported by Stefica Nicol Bikes for Reuters.com, 13 July 2021.
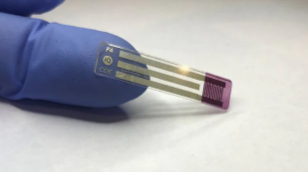 Australian scientists say they have developed the “holy grail” of blood sugar testing for diabetics, a non-invasive strip that checks glucose levels via saliva. This latest test works by embedding an enzyme that detects glucose into a transistor that can then transmit the presence of glucose, according to Paul Dastoor, Professor of Physics at the University of Newcastle in Australia, who led the team that created it. Since the electronic materials in the transistor are inks, the test can be made through printing at a low cost, Dastoor said.
Australian scientists say they have developed the “holy grail” of blood sugar testing for diabetics, a non-invasive strip that checks glucose levels via saliva. This latest test works by embedding an enzyme that detects glucose into a transistor that can then transmit the presence of glucose, according to Paul Dastoor, Professor of Physics at the University of Newcastle in Australia, who led the team that created it. Since the electronic materials in the transistor are inks, the test can be made through printing at a low cost, Dastoor said.
The new test, Dastoor said, was created by chance as scientists were working on solar cells. The project secured A$6.3 million ($4.7 million) in funding from the Australian government to establish a facility to produce the test kits should clinical trials be passed.
Read more: Australian scientists develop pain-free blood sugar test
Lilly wagers up to $1B on a biotech’s plan for a new type of insulin was published by Jonathan Gardner for BioPharmaDive.com, 14 July 2021.
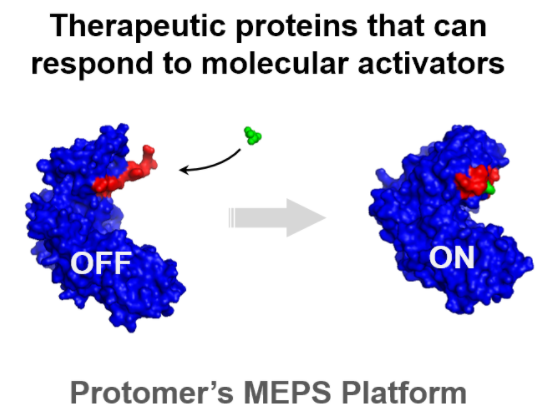 Eli Lilly will pay up to $1 billion for privately held biotech Protomer Technologies in a deal that gives the big pharma access to an experimental glucose-responsive insulin. Founded in 2015, Pasadena, Calif.-based Protomer develops drugs that respond to “molecular activators” like glucose, which is elevated to unhealthy levels in people with diabetes. Lilly joined with the Juvenile Diabetes Research Foundation to provide the startup with venture funding, and owned a 14% stake in Protomer before agreeing on Wednesday to buy the whole company.
Eli Lilly will pay up to $1 billion for privately held biotech Protomer Technologies in a deal that gives the big pharma access to an experimental glucose-responsive insulin. Founded in 2015, Pasadena, Calif.-based Protomer develops drugs that respond to “molecular activators” like glucose, which is elevated to unhealthy levels in people with diabetes. Lilly joined with the Juvenile Diabetes Research Foundation to provide the startup with venture funding, and owned a 14% stake in Protomer before agreeing on Wednesday to buy the whole company.
Diabetes researchers have sought to develop a glucose-responsive insulin since the 1970s, but they have been limited by a lack of speed and sensitivity. That could soon change, however. Novo Nordisk began a Phase 1 trial of an experimental project last year, and Lilly could keep pace through its acquisition of Protomer.
“Glucose-sensing insulin is the next frontier and has the potential to revolutionize the treatment and quality of life of people with diabetes by dramatically improving both therapeutic efficacy and safety of insulin therapy,” Ruth Gimeno, Lilly’s vice president of diabetes research and clinical investigation, said in a statement.
Read more: Lilly wagers up to $1B on a biotech’s plan for a new type of insulin
FDA devices head says progress being made on diabetes devices backlog was reported by SeekingAlpha.com, 15 July 2021.
 FDA Center for Devices and Radiological Health head Jeffrey Shuren says that while progress is being made on the number of diabetes devices the agency needs to review, a return to normalcy is expected in 2022. Shuren made the comments at the BofA Securities annual Washington D.C. Healthcare conference. The director said that COVID-19 remains a source of uncertainty for his division.
FDA Center for Devices and Radiological Health head Jeffrey Shuren says that while progress is being made on the number of diabetes devices the agency needs to review, a return to normalcy is expected in 2022. Shuren made the comments at the BofA Securities annual Washington D.C. Healthcare conference. The director said that COVID-19 remains a source of uncertainty for his division.
“Goal is to be back to normal as we roll into 2022,” Shuren said. “But there are a lot of variables that could impact that. Still not done with COVID, still get submissions, and don’t know if we’ll get hit with another tsunami of submissions for full marketing authorization for a lot of the COVID products. Getting back on track, everything is moving for the diabetes submissions.”
A number of key diabetes devices approvals and launches are expected this half and in 2022.
-
- Abbott is expected to launch Libre 3 this half of the year or in 2022
- Medtronic has 780G and Zeus CGM under review with the FDA, as well as an expected Synergy filing in H1 2022
- Insulet is expecting Omnipod 5 approval by the end of the year
- DexCom is expected to launch G7 in this half of the year
- Tandem Diabetes Care is expected to win approval of mobile bolus by the end of the year
Read more: FDA devices head says progress being made on diabetes devices backlog
FDA clears DexCom’s real-time APIs for third-party apps and devices was reported on BusinessWire.com, 15 July 2021.
 DexCom announces that the U.S. FDA has given clearance for its Dexcom Partner Web APIs. Invited third-party developers can now integrate real-time CGM data into their digital health apps and devices. The clearance expands connectivity and interoperability of the Dexcom CGM digital ecosystem. Several diabetes and digital health companies have been invited to access the real-time APIs and are already in the testing and development phase, including Garmin and Teladoc Health’s Livongo for Diabetes, DexCom said.
DexCom announces that the U.S. FDA has given clearance for its Dexcom Partner Web APIs. Invited third-party developers can now integrate real-time CGM data into their digital health apps and devices. The clearance expands connectivity and interoperability of the Dexcom CGM digital ecosystem. Several diabetes and digital health companies have been invited to access the real-time APIs and are already in the testing and development phase, including Garmin and Teladoc Health’s Livongo for Diabetes, DexCom said.“FDA clearance of our real-time APIs further solidifies Dexcom as the leader in interoperable CGM, giving Dexcom users even more choice in how they view and interact with their glucose data,” said Jake Leach, chief technology officer at Dexcom. “The new APIs will help seamlessly integrate the power of real-time Dexcom CGM data into some of the leading diabetes and digital health solutions.”
People with diabetes and their healthcare providers will benefit from the integration of real-time Dexcom CGM data into third-party apps and devices in a multitude of ways. For example, it will:
-
- Allow users to quickly see all their therapy data in one place
- Empower users to utilize the apps they find most beneficial for a more tailored Dexcom experience
- Enable in-the-moment diabetes management coaching and feedback
BIG QUESTION: Will T1Ds who very successfully use Dexcom CGM in the DIY looping apps be ALLOWED to integrate data as Dexcom Web API partners? If not, WHY NOT?
Read more: FDA Clears Dexcom Real-Time APIs for Third-Party Apps and Devices
Wave of the Future: New Glucose Technology Could Revolutionize Care was written by James Hirsch for diaTribe.org, 6 July 2021.
 For decades, researchers have sought to develop a noninvasive method to measure blood glucose levels for people with diabetes. These efforts centered on an electronic wave that could penetrate the skin, measure blood sugar, and report the number on a reader. The advantages of such a technology, if developed, were obvious: no more finger pricks for a glucose meter, no more devices attached to your abdomen or arm for a continuous glucose monitor, no more invasions, however minimal, of your body.
For decades, researchers have sought to develop a noninvasive method to measure blood glucose levels for people with diabetes. These efforts centered on an electronic wave that could penetrate the skin, measure blood sugar, and report the number on a reader. The advantages of such a technology, if developed, were obvious: no more finger pricks for a glucose meter, no more devices attached to your abdomen or arm for a continuous glucose monitor, no more invasions, however minimal, of your body.
With a PhD in physics and electronics and expertise in RF wave technology, Dr. Gerry Waintraub approached a longtime friend, retired Major General Guy Zur, who also has a background in mechanical engineering. Both men have families have been touched by diabetes. Both men recognized the potential of wave technology in diabetes care, and they reached out to two of their friends with complementary business and marketing experiences with high-tech startups: Brigadier General Bentzi Gruber and his wife, New York-born Taire Rubin. They co-founded a company in Haifa called Hagar and have developed the GWave (Glucose Wave) non-invasive monitoring system.
Gruber and Rubin compared GWave to CGM, currently the state of art in blood glucose monitoring, dominated by Dexcom’s G5 and G6 and Abbott’s FreeStyle Libre. Gruber and Rubin said that GWave is not only more convenient and less intrusive than CGM but also more accurate. GWave measures capillary blood glucose, while CGM measures interstitial fluid glucose. That’s important, because interstitial fluid glucose lags your actual blood glucose by anywhere from six to 15 minutes, which can create erroneous readings when your blood sugar is rising or falling rapidly. Additionally, the accuracy of interstitial fluid glucose can also be impaired by medications with acetaminophen, such as Tylenol. That problem, however, does not exist with GWave. The GWave also does not have to be calibrated.
Radio waves are used for other medical purposes, including magnetic resonance imaging, but in the past, wave technology failed to measure blood glucose because it could not separate out the glucose from the many other components of the blood, such as enzymes. “A lot of white noise,” Rubin said, led to inaccurate glucose readings.
GWave has solved that problem by constructing seven filters that segregate the glucose from other parts of the blood. According to Gruber, the RF technology measures a person’s blood 15 million times in a single second. Those measurements yield an average value – what Gruber calls the “X.” The system uses artificial intelligence, mathematics, and other processes to manipulate the X and determine the glucose value.
Read more: Wave of the Future: New Glucose Technology Could Revolutionize Care


I applauded the acquisition by Lilly of Protomer Technologies. I think it has a wonderful upside. Now I am not for certain it will make a big difference in insulins. But I also think it is terrific that Lilly even took the bet on Insulin. Much to be seen in the future.
I am so happy that the FDA is cutting through the backlog. I even wonder why pumps are being reviewed by FDA? Pumps are pretty much reworks of each there. I think that the main issue is the algorithm.
rick
I so totally agree with everything you’ve said!