Mixed Reactions to Dexcom’s Awareness-Raising Super Bowl Ad was written by Mike Hoskins for DiabeteMine.com, 10 Febuary 2021.
Since a 30-second spot during the game costs roughly $5.6 million, experts say many companies didn’t want to send the wrong message or opted to put the money towards COVID-19 relief instead.
There’s been some heated discussion among the Diabetes Community about whether Dexcom’s choice to invest in a Super Bowl ad was the right one — especially given the insulin pricing crisis and a record number of people with diabetes struggling to afford the treatments they need.
Yet, with roughly 100 million viewers worldwide, no doubt this ad made a huge impact in diabetes awareness across the board, with Dexcom likely hoping they’ll become a household name and that CGM will be recognized as the future standard of diabetes care.
Browsing online responses, you’ll find everything from happiness, to neutrality to outright anger — aimed at both the California CGM company as well as directly at Jonas, who despite living withT1D himself doesn’t face the same everyday struggles as most of us due to his celebrity status.
What did YOU think? Empowering? Exciting? Disrespectful? Disappointed? We’d love to hear your thoughts.
Read more: Mixed Reactions to Dexcom’s Awareness-Raising Super Bowl Ad
EMBARK Program Studies Behavioral Approaches to Reducing Diabetes Distress … interested in participating?

Interested in hearing more: EMBARK Program
The beginning of the end for Type 1 diabetes: A new view from immunotherapy science was reported on OpenAccessGovernment.org, 16 December 2021.
Imcyse SA, from the Walloon Region of Belgium, has developed a disruptive approach based on immunotherapeutic principles and logic. In December, the first patient with recent-onset Type 1 diabetes (T1D) was treated in the Company’s Phase 2 clinical trial – IMPACT – with IMCY-0098, an ImotopeTM that specifically targets the autoimmune response destroying insulin-producing cells without harming the rest of the immune system.
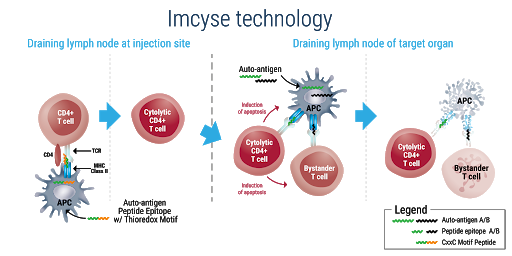 Imcyse’s new drug candidate IMCY-0098 is intended to stop the destruction of beta-cells and block the autoimmune response. Through this simple intervention, the pancreas maintains its natural ability to produce insulin. ImotopeTM science is designed as a short-term treatment regimen with the potential to provide long-term sustained effects by modifying the disease instead of only controlling the symptoms. This targeted approach has demonstrated a good safety profile observed in a Phase 1 study.
Imcyse’s new drug candidate IMCY-0098 is intended to stop the destruction of beta-cells and block the autoimmune response. Through this simple intervention, the pancreas maintains its natural ability to produce insulin. ImotopeTM science is designed as a short-term treatment regimen with the potential to provide long-term sustained effects by modifying the disease instead of only controlling the symptoms. This targeted approach has demonstrated a good safety profile observed in a Phase 1 study.
In December, in collaboration with INNODIA – a collection of T1D experts, Imcyse started a vital Phase 2 clinical trial to evaluate the ability of IMCY-0098 to stop diabetic progression in newly diagnosed patients as well as to determine the best and safe dose and regimen for continued development. The IMPACT Study is a multi-centre, clinical trial with sites located across Europe in Belgium, the United Kingdom, Sweden, Slovenia and Italy.
Read more: IMCY-0098 Proof of ACtion in Type 1 Diabetes (IMPACT Study)
All About The T1D Exchange Quality Improvement Collaborative (QIC) was discussed by Ginger Vieira for T1DExchange.org, February 2021.
 The T1D Exchange Quality Improvement Collaborative (QIC) is a network of 31 endocrinology clinics across the US, collectively caring for over 45,000 people with T1D. These clinics work together to identify areas of unmet need and use data and implementation science to make changes in clinical practice.
The T1D Exchange Quality Improvement Collaborative (QIC) is a network of 31 endocrinology clinics across the US, collectively caring for over 45,000 people with T1D. These clinics work together to identify areas of unmet need and use data and implementation science to make changes in clinical practice.
Established in 2016, the Quality Improvement Collaborative (QIC) was created by the T1D Exchange in an ambitious effort to refine best practices, enhance the quality of care, and improve outcomes for people living with T1D across the United States.
“We are proud of the QIC’s rapid growth to over 30 clinics, demonstrating that our data sharing and collaborative improvement approaches are adding real value across the T1D landscape,” said Osagie Ebekozien, Vice President of Population Health and Quality Improvement at T1D Exchange. “By working together with leading U.S. diabetes clinics, we are testing and implementing real-world solutions with benefits that can be scaled to the diabetes community.”
Read more: T1D Exchange Quality Improvement Collaborative (QIC)
New Global Diabetes Cases Ebb, as reported by Kristen Monaco for MedPageToday.com, 26 February 2021.
 Newly diagnosed diabetes cases have been slowing down worldwide, according to multinational registry data. Among 19 high-income and two middle-income countries, nearly all saw either a decline or stabilization in new diabetes cases starting in about 2010, according to Dianna Magliano, PhD, of the Baker Heart and Diabetes Institute in Melbourne, Australia, and colleagues. Out of all the locations included, the only areas that showed an uptick in new diabetes cases were Lithuania, Singapore, Israel, and the Northwestern U.S., the group wrote in the Lancet Diabetes & Endocrinology.
Newly diagnosed diabetes cases have been slowing down worldwide, according to multinational registry data. Among 19 high-income and two middle-income countries, nearly all saw either a decline or stabilization in new diabetes cases starting in about 2010, according to Dianna Magliano, PhD, of the Baker Heart and Diabetes Institute in Melbourne, Australia, and colleagues. Out of all the locations included, the only areas that showed an uptick in new diabetes cases were Lithuania, Singapore, Israel, and the Northwestern U.S., the group wrote in the Lancet Diabetes & Endocrinology.
Magliano’s group pointed out that although the root causes for the vast decline in global diabetes cases are “uncertain,” it likely points back to heightened prevention efforts. Another factor that likely impacted the rates of diagnosed diabetes cases was the formal introduction of HbA1c to diagnose diabetes — the earliest of which occurred in the U.S. in 2010.
“Importantly, the findings are limited to mostly high-income populations and cannot speak to diabetes incidence patterns in low-income and middle-income countries, where 79% of people with diabetes worldwide reside,” Ali’s group wrote.
The commentators also pointed out that the analysis only suggested that new diagnosed diabetes cases are tapering off among those with access to healthcare or those who had administrative records on their health, and therefore likely don’t count many lower socioeconomic or uninsured individuals.
Read more: New Global Diabetes Cases Ebb


Sheryl and I were discussing the super bowl ad. I am having difficulty with how I feel about it. On one hand I would not feel angst if a new type 2 medication had a super bowl ad. So why should I feel angst that Dexcom is advertising? Well i should not. Yet I do.
So Dexcom can do what they want. I know it. I endorse it. But, I don’t like it.
oh and by the way I own dexcom stock go figure
Rick … I’d swear you are my twin in so many ways! I totally agree with everything you said … and me too, we own stock! I’m doing a Zoom presentation with Dr. Francine Kaufman of Senseonics in 2 weeks … that should be interesting.