Omnipod5 app “bug” reported, 1 December 2023.
 Extracts from an email to @Insulet_UK: #Omnipod5 users describing an issue with the bolus calculator not recognizing the “.” if a bolus of less than 1u is entered without a preceding 0. For example, .5u would result in a 5u bolus, not a 1/2u bolus. Needs to be 0.5u.
Extracts from an email to @Insulet_UK: #Omnipod5 users describing an issue with the bolus calculator not recognizing the “.” if a bolus of less than 1u is entered without a preceding 0. For example, .5u would result in a 5u bolus, not a 1/2u bolus. Needs to be 0.5u. 
Link to Insulet_UK #Omnipod5 Users Forum
Kaleido launches cutting-edge Hybrid Closed Loop system with Diabeloop and Dexcom by ViCentra for PRNewsWire.com, 21 November 2023.
 ViCentra B.V., the company behind Kaleido, one of the world’s smallest, lightest insulin patch pump systems for managing type 1 diabetes, is pleased to announce the launch of a Hybrid closed-loop system for automated insulin delivery. This system delivers insulin by using the DBLG1 algorithm, which receives input from the Dexcom G6 CGM sensor.
ViCentra B.V., the company behind Kaleido, one of the world’s smallest, lightest insulin patch pump systems for managing type 1 diabetes, is pleased to announce the launch of a Hybrid closed-loop system for automated insulin delivery. This system delivers insulin by using the DBLG1 algorithm, which receives input from the Dexcom G6 CGM sensor.
Kaleido users can wear their pump as a patch or place it in their pocket at their discretion. The system comes with two durable pumps that are rechargeable, eliminating the need to dispose of the pump every 3 days as other patch pumps do. It is available in 10 bright colors and provides the ability to pause or take the pump off for intermittent breaks.
Diabeloop’s DBLG1 is a system deploying a well-known algorithm that is used by 10,000+ users in Europe already. The system draws on real-time glucose data, reducing hypoglycemia by more than 50%3 and using personalized parameters and self-learning to adjust Kaleido’s insulin delivery for optimized management of type 1 diabetes.
ViCentra is launching the Hybrid Closed Loop system in Germany, the Netherlands and France with its commercial partner Diabeloop. Roll-out to additional countries which include the UK and Italy is planned for 2024.
Read more: Kaleido launches cutting-edge Hybrid Closed Loop system with Diabeloop and Dexcom
Arizona’s Attorney General Mayes Sues Pharmacy Benefit Managers and Insulin Manufacturers Over Insulin Pricing Scheme by Richie Taylor for AZAG.gove/press-release, 27 November 2023.
 Attorney General Kris Mayes filed a lawsuit against several pharmacy benefit managers (PBMs) and pharmaceutical manufacturers for scheming to artificially inflate the price of insulin and other diabetes drugs in violation of the Arizona Consumer Fraud Act. “For the past 20 years the price of insulin increased many times faster than prices for consumer goods and services,” said Attorney General Mayes.
Attorney General Kris Mayes filed a lawsuit against several pharmacy benefit managers (PBMs) and pharmaceutical manufacturers for scheming to artificially inflate the price of insulin and other diabetes drugs in violation of the Arizona Consumer Fraud Act. “For the past 20 years the price of insulin increased many times faster than prices for consumer goods and services,” said Attorney General Mayes.
“While the drug companies and PBMs were engaged in a secretive system of baseless price increases and kickbacks, Arizona diabetes patients were facing life-or-death struggles to pay for their medication,” continued Mayes. “This lawsuit will not only force these companies to return their unfair profits to Arizona consumers but will also force the companies to operate transparently so patients can understand the true cost of their prescription drugs.”
The main PBMs named as defendants are CVS Caremark, Express Scripts, and OptumRx. The insulin manufacturers named as defendants in the lawsuit are Novo Nordisk Inc., Sanofi-Aventis U.S. LLC, and Eli Lilly and Company.
Unomedical recall of infusion sets tagged as Class I by FDA by Nick Paul Taylor for MedTechDive.com, 28 November 2023.
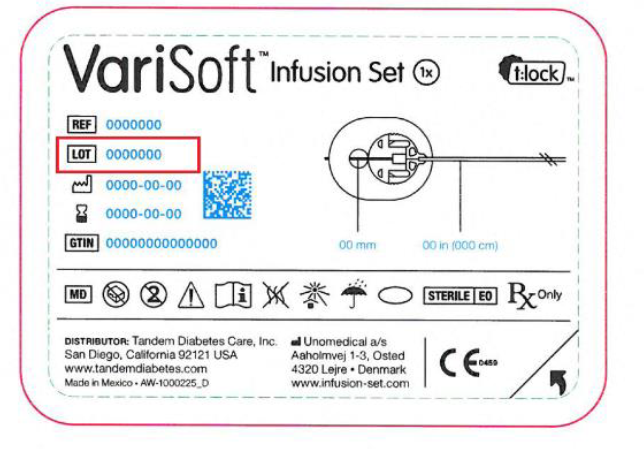 The company notified Tandem Diabetes Care, its sole consignee, in October of the risk for infusion sets to detach from insulin pumps, disrupting insulin delivery. The Food and Drug Administration has categorized Unomedical’s recall of VariSoft infusion sets as a Class I event.
The company notified Tandem Diabetes Care, its sole consignee, in October of the risk for infusion sets to detach from insulin pumps, disrupting insulin delivery. The Food and Drug Administration has categorized Unomedical’s recall of VariSoft infusion sets as a Class I event.
Unomedical, working with its partner Tandem Diabetes Care, contacted customers last month to warn them not to use certain lots of its infusion sets because of a defect that can disrupt insulin delivery. The recall affects 13,069 boxes, each of which contains 10 infusion sets, and has been linked to one reported injury and no deaths. Disrupted insulin delivery could cause hyperglycemia and potentially death.
Read more: Unomedical recall of infusion sets tagged as Class I by FDA
Arecor Therapeutics PLC Continues Collaboration with Lilly, announced by Arecor, 29 November 2023.
Under the terms of this agreement, Arecor will use its proprietary formulation technology platform, Arestat™, to develop a novel liquid formulation of one of Lilly’s products with enhanced properties. Lilly will fund the development work and has the option to acquire the rights to the new formulation and newly created intellectual property under a technology licensing model to further develop and commercialize the product. In particular, Arecor is developing AT247 (Ultra-Rapid Acting Insulin) and AT278 (Ultra-Concentrated Ultra-Rapid Acting Insulin), using their reformulation technology platform, Arestat to deliver novel formulations with enhanced properties that would otherwise be unachievable.
In particular, Arecor is developing AT247 (Ultra-Rapid Acting Insulin) and AT278 (Ultra-Concentrated Ultra-Rapid Acting Insulin), using their reformulation technology platform, Arestat to deliver novel formulations with enhanced properties that would otherwise be unachievable.
Read more: Arecore Clinical Development Programs
Insulin Manufacturers Employ Strategies to Extend Market Exclusivity by Elana Gotkine for HealthDay.com, 21 November 2023.
 Insulin manufacturers have employed strategies to extend periods of market exclusivity on brand-name insulin products, including filing patents after U.S. Food and Drug Administration approval, according to a study published online Nov. 16 in PLOS Medicine.
Insulin manufacturers have employed strategies to extend periods of market exclusivity on brand-name insulin products, including filing patents after U.S. Food and Drug Administration approval, according to a study published online Nov. 16 in PLOS Medicine.
“Our study highlights how manufacturers have listed an increasing number of patents on insulin products over the years,” coauthor William B. Feldman, M.D., also from Brigham and Women’s Hospital and Harvard Medical School, said in a statement. “These patents can delay competition and keep prices high for patients.”
To read the study: Patents and regulatory exclusivities on FDA-approved insulin products
Read more: Insulin Manufacturers Employ Strategies to Extend Market Exclusivity


From hat i have heard Diabeloop is a killer app.