Tandem agrees to buy AMF, challenging Insulet for insulin pump patch market by Nick Paul Taylor for MedTechDive.com, 14 December 2022.
 Tandem Diabetes Care agreed to acquire Switzerland-based AMF Medical to gain its insulin pump patch technology. Tandem will pay 62.4 million Swiss francs ($67 million) upfront and as much as 129.6 million francs if certain milestones are met. The deal will give Tandem control of Sigi, a reusable, rechargeable patch pump that is smaller and lighter than existing devices and integrate with continuous glucose monitors to create closed-loop systems.
Tandem Diabetes Care agreed to acquire Switzerland-based AMF Medical to gain its insulin pump patch technology. Tandem will pay 62.4 million Swiss francs ($67 million) upfront and as much as 129.6 million francs if certain milestones are met. The deal will give Tandem control of Sigi, a reusable, rechargeable patch pump that is smaller and lighter than existing devices and integrate with continuous glucose monitors to create closed-loop systems.
Read more:
Ypsomed seeks new partner for US insulin pump launch as Lilly quits deal by Nick Paul Taylor for MedTechDive.com, 12 December 2022.
 Eli Lilly has pulled out of a partnership with Ypsomed that would have given it a branded insulin pump to sell in the U.S.
Eli Lilly has pulled out of a partnership with Ypsomed that would have given it a branded insulin pump to sell in the U.S.
Lilly struck a deal two years ago to commercialize YpsoPump under its own brand in the U.S. The partners have since worked to customize the pump, which has been sold in Europe since 2016, for the U.S. market. Ypsomed plans to push ahead with the U.S. expansion without Lilly, outlining plans to file with the Food and Drug Administration in the second half of next year and commercialize the pump with a new partner.
FDA clears insulin pen medical device by Jim Carnall for LabioTech.EU, 7 December 2022.
 BioCorp, a French company specializing in the design, development, and manufacturing of innovative medical devices, has received 510(K) clearance from the U.S. Food & Drug Administration to market Mallya, its smart medical device that connects insulin pens.
BioCorp, a French company specializing in the design, development, and manufacturing of innovative medical devices, has received 510(K) clearance from the U.S. Food & Drug Administration to market Mallya, its smart medical device that connects insulin pens.
Eric Dessertenne, CEO of BioCorp, said: “This approval is a major achievement for us. This approval marks a historic achievement for BioCorp as it allows the commercial launch of our Mallya device in the United States and illustrates our ability to meet the highest regulatory requirements.
The Mallya medical device is a smart sensor that is directly attached to insulin pen injectors, making them connected devices. Mallya automatically collects and records key treatment information (selected insulin units, date, and time of injection) and transmits it to a dedicated digital application. Mallya becomes the first system approved in the U.S. capable of automatically connecting different types of insulin & GLP-1 drugs, with an initial version of Mallya compatible with SANOFI’s Solostar pen injectors.
Read more: FDA clears insulin pen medical device
Dexcom’s Jake Leach discusses preparations for G7 launch next year by Elise Reuter for MedTechDive.com, 15 December 2022.
 Dexcom’s G7 continuous glucose monitor, which the San Diego-based company expects will be its flagship device, is scheduled to launch early next year after receiving clearance from the Food and Drug Administration. The device received an expanded label, meaning it can be used by people with Type 1 and Type 2 diabetes as well as people with gestational diabetes.
Dexcom’s G7 continuous glucose monitor, which the San Diego-based company expects will be its flagship device, is scheduled to launch early next year after receiving clearance from the Food and Drug Administration. The device received an expanded label, meaning it can be used by people with Type 1 and Type 2 diabetes as well as people with gestational diabetes.
To help ensure the commercial success of the device, Dexcom has been working to make certain that insurers will include the G7 in their coverage and that the company has enough devices to meet what it expects will be a large demand, according to Chief Operating Officer Jake Leach. Dexcom plans to roll out sales of the G7 “across the globe” in 2023.
Eli Lilly and Sosei enter into diabetes and metabolic disease deal by Katherine Lewin for Endpoints.com, 16 December 2022.
 Under the agreement, Eli Lilly will pay Sosei $37 million upfront with a possibility of up to $694 million for development and commercial milestones and then tiered royalties on global sales. The pair will aim to design small molecule drugs going after G protein-coupled receptor (GPCR) targets to treat diabetes and other metabolic diseases.
Under the agreement, Eli Lilly will pay Sosei $37 million upfront with a possibility of up to $694 million for development and commercial milestones and then tiered royalties on global sales. The pair will aim to design small molecule drugs going after G protein-coupled receptor (GPCR) targets to treat diabetes and other metabolic diseases.
GPCRs, a group of membrane proteins, are among the most prolific target classes in all of the drug discovery and have been the focus of hundreds of approved medicines over several decades. Eli Lilly nominated several target-selective small molecule candidates for Sosei to focus on as R&D continues.
Read more: Eli Lilly and Sosei enter into diabetes and metabolic disease deal
Researchers identify insulin-mimicking molecule that could treat diabetes by Clarissa Brincat for MedicalNewsToday.com, 16 December 2022.
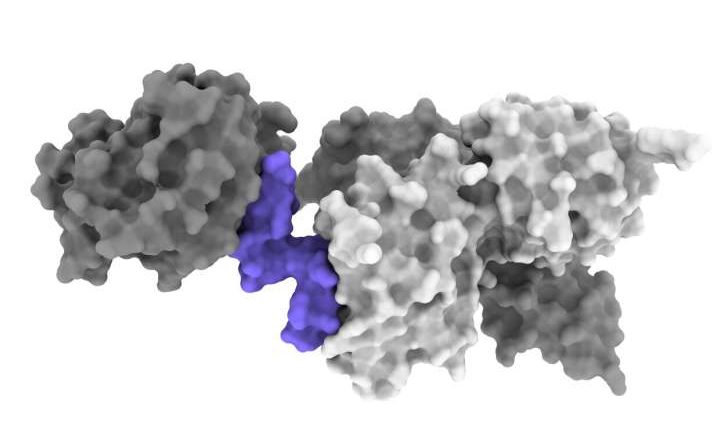 Researchers at the Walter and Eliza Hall Institute (WEHI) in Melbourne, Australia, in collaboration with researchers at Lilly, have identified a protein that mimics the role of insulin, potentially paving the way for the development of an oral pill to replace insulin injections. Their findings were recently published in Nature Communications
Researchers at the Walter and Eliza Hall Institute (WEHI) in Melbourne, Australia, in collaboration with researchers at Lilly, have identified a protein that mimics the role of insulin, potentially paving the way for the development of an oral pill to replace insulin injections. Their findings were recently published in Nature Communications
The obstacle to oral insulin administration is the fact that insulin is a protein that is digested in the stomach before it reaches the bloodstream. For insulin to work, it must get past the stomach and into the bloodstream still intact, to be transported to the insulin receptors on fat, muscle, and liver cells. Daniel J. Leahy, Ph.D., professor of molecular biosciences at the University of Texas at Austin, noted: “It has been difficult to make an insulin-supplanting pill as insulin is a protein that would be digested in the gut, and any insulin-mimicking ‘pill’ would have to survive being digested as well as be absorbed into the bloodstream.”
In addition, recent breakthroughs in cryo-electron microscopy (cryo-EM), a technique for determining the 3D shape of biomolecules, have enabled researchers to visualize complicated molecules like insulin in great detail. Cryo-EM involves flash-freezing solutions of biomolecules and then bombarding them with electrons to produce magnified images of individual molecules. Using cryo-EM, researchers are able to generate 3D images of the insulin receptor and observe how insulin and other molecules alter its shape.
Nicholas Kirk, Ph.D., study first author and senior research officer at WEHI, described the interaction between insulin and the receptor as being “like a hand bringing a pair of tongs together.” Certain peptides (short chains of amino acids linked together by peptide bonds) are known to interact with the insulin receptor in a similar manner to insulin. The researchers used cryo-EM to examine the interaction between these peptides and the insulin receptor at a molecular level. They found that a specific peptide consisting of a chain of 33 amino acids can bind to and activate the insulin receptor in a similar manner to insulin.
Read more:
U.S. FDA flags shortage of Eli Lilly’s new diabetes drug Mounjaro posted by Reuter.com, 16 December 2022.
 The U.S. health regulator has added Eli Lilly and Co’s Mounjaro to its list of drugs facing shortages, highlighting the company’s struggles to meet booming demand for the newly approved diabetes injection. Trulicity, another diabetes treatment in the company’s stable and its biggest-selling drug, was also added to the Food and Drug Administration’s shortage list on Thursday.
The U.S. health regulator has added Eli Lilly and Co’s Mounjaro to its list of drugs facing shortages, highlighting the company’s struggles to meet booming demand for the newly approved diabetes injection. Trulicity, another diabetes treatment in the company’s stable and its biggest-selling drug, was also added to the Food and Drug Administration’s shortage list on Thursday.
The additions come as Lilly earlier this week flagged challenges to meet the demand for the two drugs, especially as it makes six dosage forms of Mounjaro and four of Trulicity. The company is expanding its manufacturing capacity for the two drugs through its facility in North Carolina and doubling it by the end of 2023.
Trulicity generated $5.5 billion this year through Sept. 30, but the company and investors have pinned their hopes on Mounjaro to drive future growth. Supply problems have also plagued a rival obesity drug, Wegovy, from Novo Nordisk, although the Danish drugmaker is also working on boosting manufacturing capacity.
Read more: FDA flags shortage of Eli Lilly’s new diabetes drug Mounjaro
Further observations of Dexcom’s G7 by Tim Street on Diabettech.com, 15 December 2022. Does this cause you concern about the Dexcom G7???
Having tested a G7 against the Libre3, and found that without calibration, it tended to produce results that I wasn’t satisfied with using just the factory calibration, I thought I’d have another go and see where we got to. This is one that was ordered from the UK website, and applied according to the user guide on my arm. The reference data recording wasn’t as methodical as the capture for the previous study, but the results were very far from what I was expecting.
What’s going on?

I’m not sure. This is the second device that I’ve used with readings that were a long way off when using the factory calibration, and also the second that fell off early (one with and one without the overlay patch). I submitted an issue and have a replacement sensor coming, which I’ll check again. If I still see the same problems, then I assume that I don’t get on with the G7 as well as I did the G6.
One of the major differences between the G7 and the G6 is the orientation of the sensor wire, which is now vertical, compared to the angled entry of the G6. It’s possible that this could be interacting with me in a way that causes movement within the “sheath” and affects the results.
Having said all of that, anecdotal observation of the G6 and ONE systems that I have historically used has also recently shown a higher level of variation than I have been used to with the factory calibration. At least once in the life of a sensor, I am now checking and calibrating. Something seems to have changed, but I can only assume it’s me, as I can’t imagine that the devices are being manufactured or set up differently.
Read more: Further observations of Dexcom’s G7


Lots of Eli Lilly news today. I am shocked that they abandoned the deal with Ypsomed. Everything I had heard is that Lilly was close to early trials on the modified pump. Something (I am guessing marketability) It would be fascinating to know what happened.