Beta Bionics Announces Closing of $57 Million Series C Financing as reported on GlobeNewswire.com, 17 February 2022.
 Beta Bionics, Inc. — a clinical stage medical technology company committed to the design, development, and commercialization of the iLet® Bionic Pancreas System — announced the completion of a $57 million Series C equity financing. Proceeds from the financing will support product development, regulatory submissions, and preparations for the commercialization of the insulin dosing iLet® Bionic Pancreas System following FDA clearance.
Beta Bionics, Inc. — a clinical stage medical technology company committed to the design, development, and commercialization of the iLet® Bionic Pancreas System — announced the completion of a $57 million Series C equity financing. Proceeds from the financing will support product development, regulatory submissions, and preparations for the commercialization of the insulin dosing iLet® Bionic Pancreas System following FDA clearance.
The Series C financing round was co-led by existing Series B and B-2 investors Soleus Capital, Perceptive Advisors, Farallon Capital Management, L.L.C., RTW Investments, LP, and Eventide Asset Management. In addition, new investor Pura Vida Investments participated in the round, as did previous Series B and B-2 investors ArrowMark Partners, LifeSci Venture Partners, and strategic partner Novo Nordisk. This financing adds to the Series B and B-2 financings in 2018 and 2019 that raised approximately $126 million.
“We are very pleased to have completed our third round of institutional financing and are excited to welcome Pura Vida into our strong syndicate of investors,” said Dr. Ed Damiano, President and CEO, Beta Bionics. “This financing will support the growth of our organization and further development of the iLet, as we continue to work toward our mission to bring autonomous insulin-delivery solutions to those living with type 1 diabetes. We are grateful for the continued support and vision of our investor syndicate and are delighted to be adding another well-respected investor to this esteemed group.”
“We are excited to play a role in helping Beta Bionics deliver patient-centric innovation to the type 1 diabetes community and reduce the cognitive and emotional impacts that type 1 diabetes has on this population,” said Efrem Kamen, managing member of Pura Vida Investments. “We look forward to supporting the team and company through the next phase of its growth.”
Read more: Beta Bionics Announces Closing of $57 Million Series C Financing
Scanbo: Noninvasive Gadget Scans Your Fingers to Measure Blood Glucose was written by Mike Hoskins for DiabetesMine.com, 6 February 2022.
 Founded in late 2017, Scanbo, based in British Columbia, Canada, is a young medtech company is led by Ashissh Raichura, who has a background in IT consulting and entrepreneurship, focused lately on artificial intelligence (AI) software. As the name suggests, Scanbo’s concept is about scanning your skin. In this case, your fingertips are placed on a small digital pad and the device uses a proprietary algorithm to assess your glucose levels.
Founded in late 2017, Scanbo, based in British Columbia, Canada, is a young medtech company is led by Ashissh Raichura, who has a background in IT consulting and entrepreneurship, focused lately on artificial intelligence (AI) software. As the name suggests, Scanbo’s concept is about scanning your skin. In this case, your fingertips are placed on a small digital pad and the device uses a proprietary algorithm to assess your glucose levels.
The device is still in prototype stage, but is interesting because it takes a different approach than competitors, combining electrocardiogram (ECG) measurement with optical photoplethysmogram (PPG) sensing for the first time to measure blood glucose levels. “This will be a blessing for prediabetics who are not comfortable poking their fingers multiple times in a day, and do not want to spend money on glucose strips and costly continuous monitoring devices,” said Raichura.
This is not a wearable device or patch, but is rather more similar to a traditional glucose meter that you buy and have on hand to use as needed. It is a small handheld pad that folds in half, with the fingertip scanning portion on the lower end and the screen showing data results at the top. It collects data using two different measurements, most often used for monitoring heart rates:
-
- ECG, which typically uses small, plastic patch electrodes that are stuck to the skin on the chest, arms, and legs, and then those electrodes are connected to an ECG machine by lead wires.
- PPG, non-invasive technology that uses a light source and a photo-detector at the surface of skin to measure the volumetric variations of blood circulation.
Read more: Scanbo: Noninvasive Gadget Scans Your Fingers to Measure Blood Glucose
2021 Diabetes Atlas Numbers was reported by Natalie Sainz for diaTribeChange.org, 18 February 2022.
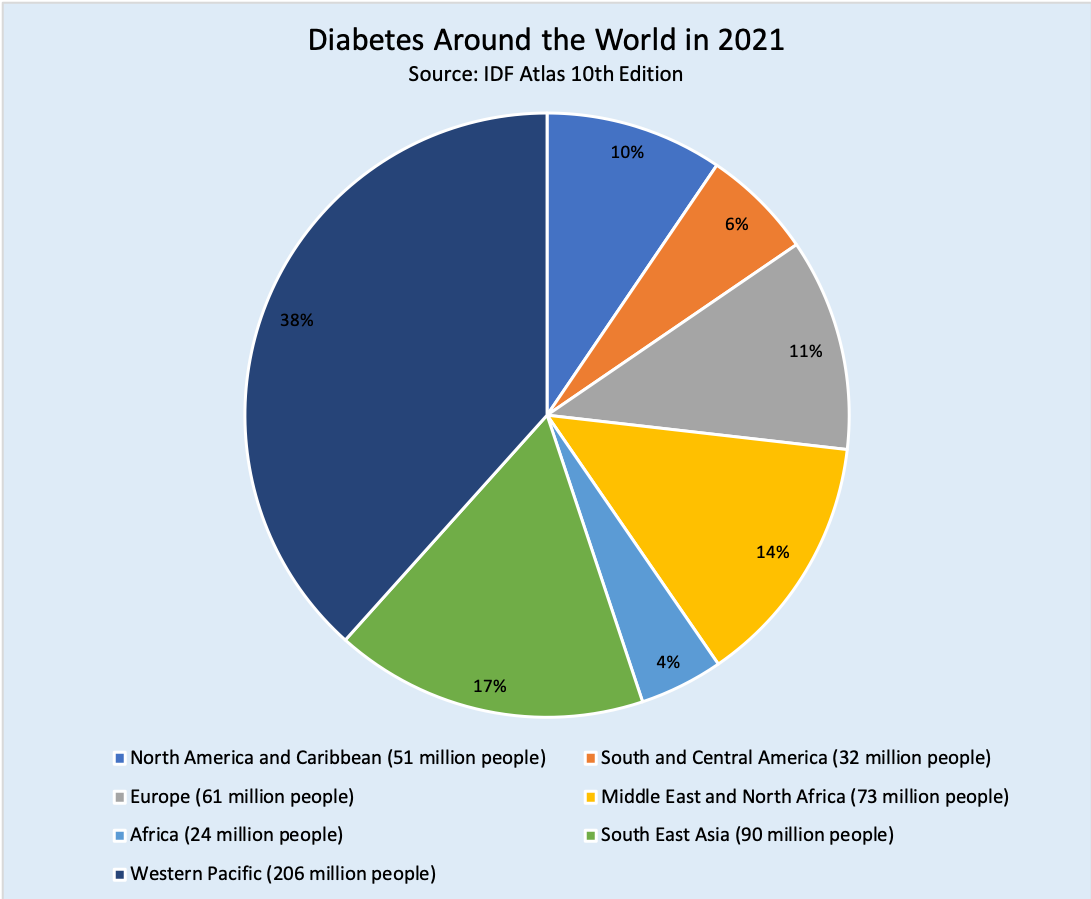
The International Diabetes Federation (IDF) released their 10th edition of the IDF Diabetes Atlas, an annual report of global estimates of diabetes prevalence, mortality, and related health expenditures in 2021. Although most people with diabetes, as well as private and public payers (including governments), are spending more money than ever on preventing and treating diabetes, rates of diabetes prevalence and mortality continue to grow worldwide.
“I wish I could report that the past two decades have witnessed decisive action to tackle diabetes and that the rising tide of diabetes has finally turned,” said Andrew Boulton, IDF president, outlining the dire findings of the newly updated Diabetes Atlas. “Rather, I must repeat the message that diabetes is a pandemic of unprecedented magnitude spiraling out of control.”
These are some key findings from the report:
-
- Prevalence: Globally, more than 1 in 10 adults aged 20-79 years (over half a billion people) now live with diabetes (both type 1 and type 2). This number has more than tripled since 2000 from an estimated 151 million to 537 million today, including more than 1.2 million children and adolescents who have type 1 diabetes.
- COVID-19: During the first wave of COVID-19, people with diabetes had a 3.6-fold higher likelihood of being hospitalized due to infection, compared to those without diabetes.
- Health Expenditure: The global health expenditure of diabetes was USD $966 billion – a 316% increase over the last 15 years.
Read more: 2021 Diabetes Atlas Numbers
Mimicking natural light cycles may influence diabetes risk was written by Mary McGorray, MD for MedicalNewsToday.com, 22 February 2022.
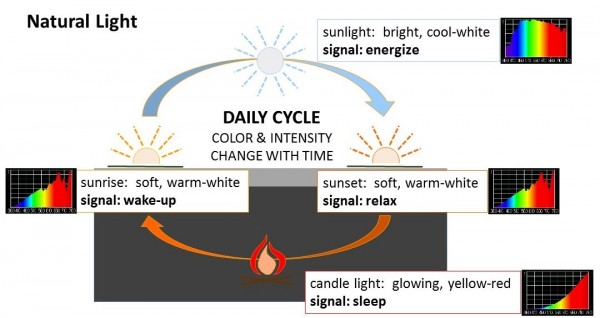 Recently, a team of researchers theorized that societal changes such as increased exposure to artificial light might be one factor linked to the global rise in people with insulin resistance. As reported in the journal Diabetologia, Prof. Patrick Schrauwen, Jan-Frieder Harmsen, and their colleagues tested their hypothesis by studying individuals who already had insulin resistance.
Recently, a team of researchers theorized that societal changes such as increased exposure to artificial light might be one factor linked to the global rise in people with insulin resistance. As reported in the journal Diabetologia, Prof. Patrick Schrauwen, Jan-Frieder Harmsen, and their colleagues tested their hypothesis by studying individuals who already had insulin resistance.
Scientists discovered several interesting results:
-
- Melatonin release was significantly suppressed in the dim day-bright evening scenario.
- Those who spent the day in bright light had lower glucose levels before dinner and higher metabolic rates at night.
- Metabolism slowed after dinner in individuals in the dim day-bright evening scenario.
- In people who spent the day in bright light with a dim night, melatonin levels were increased at night, which is helpful for inducing sleep.
The researchers concluded that the timing of light exposure can influence the body’s handling of glucose and fats, energy use and expenditure, and even temperature regulation in insulin-resistant individuals. Insulin resistance occurs when cells do not respond to insulin as efficiently, reducing their ability to take in glucose from the blood.
Read more: Mimicking natural light cycles may influence diabetes risk
CMS code seen as major step toward reimbursement for digital therapeutics was reported by Elise Reuter for MedTechDive.com, 25 February 2022.
 A new CMS code would make it easier for digital therapeutics companies to get reimbursed for their software-based treatments. The CMS published a new Level II Healthcare Common Procedure Coding System (HCPCS) code for “prescription digital behavioral therapy,” which could make it easier for commercial and Medicaid plans to cover these therapies. It will go into effect in April. Although more digital therapeutics are getting FDA indications, companies are still figuring out what payment models work best.
A new CMS code would make it easier for digital therapeutics companies to get reimbursed for their software-based treatments. The CMS published a new Level II Healthcare Common Procedure Coding System (HCPCS) code for “prescription digital behavioral therapy,” which could make it easier for commercial and Medicaid plans to cover these therapies. It will go into effect in April. Although more digital therapeutics are getting FDA indications, companies are still figuring out what payment models work best.
HCPCS codes would serve as another option for payers to cover its digital therapeutics as a medical device. There are also plans to put forth legislation that would create a specific benefit type for digital therapeutics through the CMS.
Today’s HCPCS code is a nice example of how CMS views PDTs [prescription digital therapeutics] as a discrete product type, and we may not even need the legislation ultimately.
American Medical Association last year clarified its CPT code for remote monitoring to include cognitive behavioral therapy, which would allow clinicians to be reimbursed for time spent checking patients’ progress through dashboards. It’s expected to go into effect in 2023.
Read more: CMS code seen as major step toward reimbursement for digital therapeutics
Vertical integration secures PBMs as ‘arsonists and firefighters’ of drug prices was written by Rob Volansky for Healio.com/endocrinology, 22 February 2022.
 In the last 4 years, the three biggest pharmacy benefit managers have consolidated influence in the U.S. health care marketplace through vertical integration with insurance carriers. These transactions have contributed as much, if not more, to skyrocketing prescription drug costs as the pharmaceutical industry itself, according to experts.
In the last 4 years, the three biggest pharmacy benefit managers have consolidated influence in the U.S. health care marketplace through vertical integration with insurance carriers. These transactions have contributed as much, if not more, to skyrocketing prescription drug costs as the pharmaceutical industry itself, according to experts.
“At the end of 2018, we saw the final vertical integration of a large insurance company and a PBM, with the purchase of Aetna by CVS Caremark,” Madelaine A. Feldman, MD, FACR, president of the Coalition of State Rheumatology Organizations, founder and past president of the Rheumatology Alliance of Louisiana and a chair of the Alliance for Safe Biologic Medicines, told Healio Rheumatology. “Cigna purchasing Express Scripts had gone through, and of course, we know United Healthcare has already owned OptumRx.”
Vertical integration, simply put, is when a company owns its own supply chain. The classic example is Andrew Carnegie and the Carnegie Steel Company. Founded in 1892, the company controlled not only the steel mills, but also the mines that supplied the necessary iron ore and coal, as well as the ships and railroads that transported them. Shifting this strategy to prescription drugs, the three largest PBMs — UnitedHealth Group’s OptumRx, CVS Health Corp’s CVS Caremark and Cigna’s Express Scripts — now process nearly 80% of all prescription claims in the United States.
Harry L. Gewanter, MD, director for CSRO, medical director of Medical Home Plus, and clinical associate professor of pediatrics at Children’s Hospital of Richmond at Virginia Commonwealth University, was more pointed. “PBMs are like a weed that has infiltrated the entire soil of our health care system,” he said. “They keep coming up with new ways to make it harder to track where the money for drugs is going.”
Read more: Vertical integration secures PBMs as ‘arsonists and firefighters’ of drug prices
A first here … a TikTok lesson on CRISPR which was used in the first patient treated in Phase 1 clinical trial for treatment of Type 1 diabetes.
@crisprclassroom Reply to @nz_russ CRISPR x Diabetes #crisprclassroom #crispr #diabetes #genetics #geneticdisorder #sciencetok #STEMtok #sciencetokers


Just a note on PBM’s. When I see groups protesting at the Lilly Headquarters over insulin prices, I always ask, why? The issue is the PBM’s. Why protest, our co-victims?