Insulet gains amid winning TRO in dispute with EOFlow, CEO insider buy by Joshua Fineman for SeekingAlpha.com, 30 August 2023.
 Insulet rose 8.1% at least partly after the insulin pump maker was granted a temporary restraining order in a trade secrets case against rival EOFlow, which is being acquired by Medtronic. A judge issued a TRO to stop EOFlow from sharing disputed information related to the EOPatch or Ominpod products with any third parties. The restraining order remains in effect until Sept. 12, though it can be extended.
Insulet rose 8.1% at least partly after the insulin pump maker was granted a temporary restraining order in a trade secrets case against rival EOFlow, which is being acquired by Medtronic. A judge issued a TRO to stop EOFlow from sharing disputed information related to the EOPatch or Ominpod products with any third parties. The restraining order remains in effect until Sept. 12, though it can be extended.
The case is important because some believe that Insulet filed the lawsuit against competitor EOFlow to potentially hurt Medtronic’s chances of acquiring EOFlow in a $738 million deal announced in May. Insulet shares fell 5.7% on May 25 when the Medtronic deal was announced and peer Tandem Diabetes dropped 6.7% the same day. The implications of the TRO are small, as Medtronic likely has already reviewed the information it needs for due diligence since the deal was announced in May. Secondly, the ruling here is mainly designed to buy the court some time, according to an analyst.
Read more: Insulet gains amid winning TRO in dispute with EOFlow
Beta Bionics raises $100M to challenge Medtronic, Tandem for automated insulin dosing market by Nick Paul Taylor for MedTechDive.com, 1 September 2023.
 Beta Bionics raised a $57 million series C financing round early last year. That financing, which was co-led by a syndicate of venture capital funds with an assist from strategic partner Novo Nordisk, equipped the company to develop iLet, file for FDA clearance, and prepare to commercialize the device. Eighteen months later, Beta Bionics has executed the strategy it outlined at the time of the series C, and persuaded investors to bankroll the next stage of its evolution. New investors Sands Capital and Omega Funds co-led the series D round. British hedge fund Marshall Wace and existing investors such as Soleus Capital, Eventide Asset Management, Farallon Capital and Perceptive Advisors also contributed cash.
Beta Bionics raised a $57 million series C financing round early last year. That financing, which was co-led by a syndicate of venture capital funds with an assist from strategic partner Novo Nordisk, equipped the company to develop iLet, file for FDA clearance, and prepare to commercialize the device. Eighteen months later, Beta Bionics has executed the strategy it outlined at the time of the series C, and persuaded investors to bankroll the next stage of its evolution. New investors Sands Capital and Omega Funds co-led the series D round. British hedge fund Marshall Wace and existing investors such as Soleus Capital, Eventide Asset Management, Farallon Capital and Perceptive Advisors also contributed cash.
The new investment “represents a powerful vote of confidence in Beta Bionics’ mission,” CEO Sean Saint said in a statement on Wednesday. He added that the company is eager to expand access nationwide and “further develop and test the bi-hormonal bionic pancreas.”
Read more: Beta Bionics raises $100M to challenge Medtronic, Tandem for automated insulin dosing market
$2M Grant Drives Research on Novel Insulin in Type 1 Diabetes by Sara Bock, Today.UCSD.edu, 23 August 2023.
 Jeremy Pettus, MD, an associate professor of medicine and board-certified endocrinologist who specializes in treating diabetes at UC San Diego Health, is leading a study on Liver Targeted Insulin (LTI) in Type 1 diabetes. The Leona M. and Harry B. Helmsley Charitable Trust recently provided a grant of more than $2 million to UC San Diego to support the research effort.
Jeremy Pettus, MD, an associate professor of medicine and board-certified endocrinologist who specializes in treating diabetes at UC San Diego Health, is leading a study on Liver Targeted Insulin (LTI) in Type 1 diabetes. The Leona M. and Harry B. Helmsley Charitable Trust recently provided a grant of more than $2 million to UC San Diego to support the research effort.
Pettus and a team of researchers are working to determine the mechanism of action and evaluate the safety profile of the Liver Targeted Insulin. Directing insulin action to the liver may help restore normal liver physiology for people with Type 1 diabetes, leading to better glucose control and overall health outcomes.
“Living with Type 1 diabetes is extremely tough,” said Pettus. “One of the major barriers to helping patients with Type 1 diabetes achieve normal glucose levels is that injected insulin simply does not get to the ‘right’ places. Normal insulin has its main effects in the liver, but patients with Type 1 diabetes must inject insulin into the fat tissue. Doing so makes the insulin act very slowly, can lead to weight gain and can cause deadly low blood sugars. This project seeks to attach a molecule to insulin that can help direct it to the liver. In doing so, much of the normal physiology may be restored and patients may get better results.”
Pettus is working with the company Diasome to conduct a single-center clinical study with 14 patients with Type 1 diabetes to test the safety, tolerability and effectiveness of the novel LTI.
Read more:
A DIY ‘bionic pancreas’ is changing diabetes care — what’s next? by Liam Drew for Nature.com, 30 August 2023. A community of people with type 1 diabetes got a self-built device approved. What can they offer that big companies can’t? AN OUTSTANDING article about the DIY automated “pancreas” and what’s next!
Ten years ago, a tech-savvy group of people with type 1 diabetes (T1D) decided to pursue a DIY approach to their own treatment. They knew that a fairly straightforward piece of software could make their lives much easier, but no companies were developing it quickly enough. What this software promised was freedom from having to constantly measure and control their blood glucose levels. “It is almost inhumane,” says Shane O’Donnell, a medical sociologist at University College Dublin, who, like everyone quoted in this article, lives with T1D. “You’re constantly having to think about diabetes in order to survive.”
 Members of the nascent DIY community were using the most sophisticated technology available: insulin pumps and wearable devices called constant glucose monitors. But they still had to read the monitor’s data, forecast their diet and exercise, and then calculate the appropriate insulin dose. What they wanted was automation — an algorithm that would analyze glucose data and program the pump itself. Coalescing around this aim in 2013, the community debuted a hashtag: #WeAreNotWaiting.
Members of the nascent DIY community were using the most sophisticated technology available: insulin pumps and wearable devices called constant glucose monitors. But they still had to read the monitor’s data, forecast their diet and exercise, and then calculate the appropriate insulin dose. What they wanted was automation — an algorithm that would analyze glucose data and program the pump itself. Coalescing around this aim in 2013, the community debuted a hashtag: #WeAreNotWaiting.
Then, in February 2015, group member Dana Lewis shared the code for an algorithm that she and two collaborators had developed and tested. Katarina Braune, an endocrinologist at Charité – Berlin University Medicine, estimates that around 30,000 people now use open-source technology for automated insulin delivery (AID). Some use Lewis and colleagues’ original OpenAPS system, which requires a minicomputer to control it, whereas others use either AndroidAPS (which evolved from Lewis’ system) or Loop, which are smartphone applications.
The movement has continued to mature. After years of relying on self-reported data, in the past year, two randomized controlled trials have shown the safety and effectiveness of open-source systems. In January 2023, the US Food and Drug Administration (FDA) granted regulatory clearance to an AID system based on an open-source algorithm for the first time.
Is this the beginning of the end for the open-source movement in diabetes care? Some diabetologists think so. But many advocates reject that idea, saying that the community is still pushing the technology in new directions that promise more personalization and automation than commercial versions currently provide.
Read more:
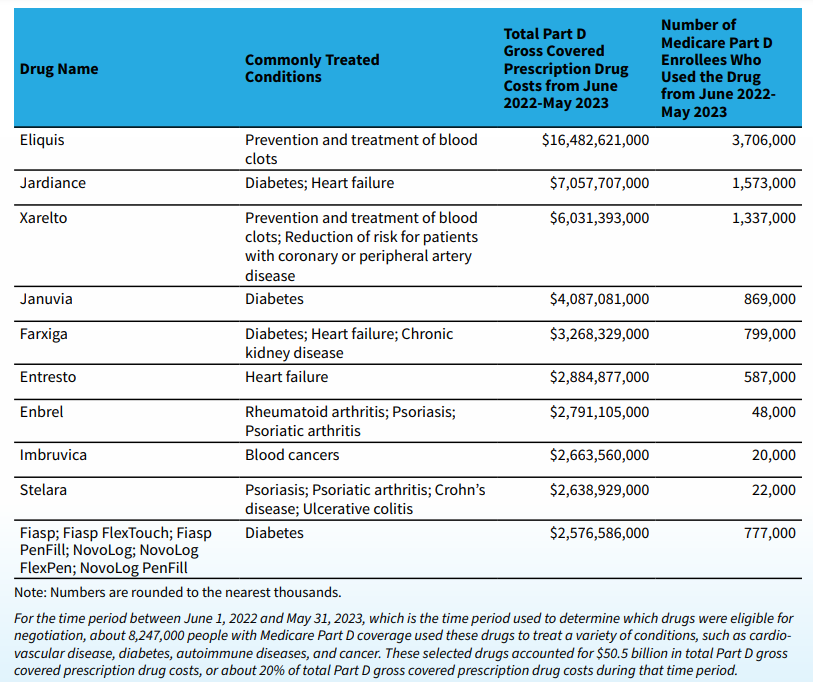
Medicare names first 10 drugs for price negotiations by Ned Pagliarulo for BioPharmaDive.com, 29 August 2023.
Medicare will negotiate the prices of top-selling drugs for blood clots, diabetes, cancer and arthritis, flexing newly granted authority that for the first time allows the insurance program to use its market power to lower the cost of certain medicines. Here is the list of 10 drugs that will be included in the first round of negotiations, which will run over the next year and produce prices that take effect in 2026.
Read more: Medicare names first 10 drugs for price negotiations
Welldoc Receives 11th 510(k) Clearance from FDA for Award-Winning Diabetes Platform BlueStar® in their press release, 23 August 2023.
 Welldoc, a digital health leader revolutionizing chronic care, announced the receipt of its 11th 510(k) clearance from the Food and Drug Administration (FDA) for its award-winning diabetes digital health solution, BlueStar, which enables BlueStar to provide bolus insulin dose recommendations based on the most recent glucose reading and rate of change from a compatible continuous glucose monitoring (CGM) device. This feature enhances BlueStar’s existing digital coaching capabilities, which guide dietary and lifestyle decisions and assist individuals in self-managing their diabetes. Welldoc is the first company to receive clearance for a CGM-informed bolus calculator specifically designed for adults who manage their diabetes with multiple daily injections of insulin.
Welldoc, a digital health leader revolutionizing chronic care, announced the receipt of its 11th 510(k) clearance from the Food and Drug Administration (FDA) for its award-winning diabetes digital health solution, BlueStar, which enables BlueStar to provide bolus insulin dose recommendations based on the most recent glucose reading and rate of change from a compatible continuous glucose monitoring (CGM) device. This feature enhances BlueStar’s existing digital coaching capabilities, which guide dietary and lifestyle decisions and assist individuals in self-managing their diabetes. Welldoc is the first company to receive clearance for a CGM-informed bolus calculator specifically designed for adults who manage their diabetes with multiple daily injections of insulin.
“With this clearance, Welldoc is filling a significant gap for people who require complex insulin regimens. By connecting directly with CGM data and using both glucose values and trend arrows, the BlueStar solution will provide precise and in-the-moment insulin dosing guidance directly to individuals, helping them reach their glucose targets,” said Dr. Grazia Aleppo, a practicing endocrinologist, with the Feinberg School of Medicine at Northwestern University as well as a principal investigator in Welldoc’s clinical validation study for this product.
Read more: Welldoc Receives 11th 510(k) Clearance from FDA for Award-Winning Diabetes Platform BlueStar®
Before a cardiac arrest, men and women have different symptoms by Corrie Pelc for MedicalNewsToday.com, 31 August 2023.
 “If you have diabetes, you’re twice as likely to have heart disease or a stroke than someone who doesn’t have diabetes—and at a younger age. The longer you have diabetes, the more likely you are to have heart disease” according to the Centers for Disease Control and Prevention: Diabetes and Your Heart
“If you have diabetes, you’re twice as likely to have heart disease or a stroke than someone who doesn’t have diabetes—and at a younger age. The longer you have diabetes, the more likely you are to have heart disease” according to the Centers for Disease Control and Prevention: Diabetes and Your Heart
Of the more than 356,000 cardiac arrests in the United States each year, 90% are fatal. While there are some known signs of sudden cardiac arrest, it usually occurs without warning. Researchers from the Smidt Heart Institute at Cedars-Sinai Health System have found that half of people experiencing a sudden cardiac arrest also had a telling symptom 24 hours beforehand. Scientists also discovered those warning symptoms are different between men and women.
Cardiac arrest occurs when the heart suddenly stops beating, stopping blood from pumping throughout the body. The main cause of sudden cardiac arrest is an arrhythmia or abnormal heartbeat. An arrhythmia occurs when the electrical pulses telling the heart to pump blood are disrupted. Cardiac arrest is different from a heart attack. A heart attack happens when a blockage in an artery stops blood from flowing through the different sections of the heart, but it does not cause the heart to completely stop beating as cardiac arrest does.
Cardiac arrest warning signs: Men vs. women: The scientists also found differences in the sudden cardiac arrest warning symptoms between men and women. Researchers found that
-
-
- For women 24 hours before cardiac arrest, shortness of breath is the primary symptom
- For men, chest pain was the preeminent telltale symptom of cardiac arrest
-
“There is increasing and urgent recognition of how important it is to always perform clinical research that can evaluate sex differences between conditions,” Dr. Sumeet Chugh, a cardiac electrophysiologist and director of the Center for Cardiac Arrest Prevention said.
Read more: Before a cardiac arrest, men and women have different symptoms
Why medtech firms are putting diabetes tech in consumer devices by Elise Reuter for MedTechDive.com, 31 August 2023.
 In a year marked by the launch of new diabetes devices, Abbott and Dexcom announced plans for new sensors intended for people who don’t take insulin. Abbott Laboratories debuted a new wearable in July designed to help people without diabetes track glucose spikes and lows. The company, which makes continuous glucose monitors to help people manage their diabetes, quietly rolled out a new website for the consumer-facing device called Lingo, which is currently available in the U.K.
In a year marked by the launch of new diabetes devices, Abbott and Dexcom announced plans for new sensors intended for people who don’t take insulin. Abbott Laboratories debuted a new wearable in July designed to help people without diabetes track glucose spikes and lows. The company, which makes continuous glucose monitors to help people manage their diabetes, quietly rolled out a new website for the consumer-facing device called Lingo, which is currently available in the U.K.
Abbott is billing the device as “Your personal metabolic coach for optimal wellbeing,” as part of a subscription package costing 150 pounds ($190) per month. Users receive two sensors, similar to Abbott’s Freestyle Libre CGMs, but the resulting data is presented in a different way through an app, with a focus on counting glucose spikes throughout the day.
Abbott CEO Robert Ford has said he expects the consumer devices to contribute to the company’s goal of $10 billion in sales for its Libre business by 2028. fter testing Lingo in the U.K., Abbott plans to roll it out to other markets, including filing in the U.S. at the end of the year.
Competitor Dexcom is also looking for ways to extend its sensor technology to a broader group of people. The company announced plans in June to develop a new CGM targeted at people with Type 2 diabetes who don’t take insulin. “We envision that there will be multiple iterations and generations of this product focused on adults who are not on insulin,” Dexcom Chief Commercial Officer Teri Lawver said in an interview.
Read more:


I am so excited to be one of the cool kids with D tech. Who knew 49 years ago every one would want to be with the D!!
I am all in on medicare drug negotiation. I know several groups I am part of are warning against the process, but i do not fear it. I welcome it. we will see I guess.