In this week’s issue of The Savvy Diabetic:
-
- 3D-Printed Islet Transplantation
- Abvance Therapeutics Funded for Next-Gen Glucagon Analogs/Therapies to Prevent Hypos
- Glucotrack’s CBGM Implant Tech
- Ax-4 Space Mission Studies Glucose Metabolism & Microgravity
- Study: Implementation of AID Systems, FDA’s Interoperability Designation
3D Printing Could Enable Long-Term Islet Transplantation in Type I Diabetes by InsidePrecisionMedicine.com, 30 June 2025.
 Researchers have developed a new method to 3D print functional human islets that can sustain strong insulin responses for up to three weeks. Using a novel bioink derived from human pancreatic tissue could unlock an innovative, minimally invasive approach to islet transplantation, potentially overcoming some of the significant challenges facing the development of cell therapies for type 1 diabetes.
Researchers have developed a new method to 3D print functional human islets that can sustain strong insulin responses for up to three weeks. Using a novel bioink derived from human pancreatic tissue could unlock an innovative, minimally invasive approach to islet transplantation, potentially overcoming some of the significant challenges facing the development of cell therapies for type 1 diabetes.
“This is one of the first studies to use real human islets instead of animal cells in bioprinting, and the results are incredibly promising. It means we’re getting closer to creating an off-the-shelf treatment for diabetes that could one day eliminate the need for insulin injections,” said Quentin Perrier, PhD, researcher at the Wake Forest University School of Medicine, who presented the findings this weekend at the European Society for Organ Transplantation (ESOT) Congress 2025.
The new method developed by Perrier and colleagues has shown early promise to lengthen the time transplanted islets can remain functional. “Our goal was to recreate the natural environment of the pancreas so that transplanted cells would survive and function better,” said Perrier. “We used a special bioink that mimics the support structure of the pancreas, giving islets the oxygen and nutrients they need to thrive.”
The innovation lies in the composition of the 3D printing bioink. The extracellular matrix (ECM) is known to play an essential role in the survival and function of pancreatic islets; however, current methods for isolating cells for islet transplantation strip it away. The researchers developed a gentle, detergent-free method to obtain a soluble ECM powder from human pancreas, retaining key components that support the survival and function of islets.
Read more: 3D Printing Could Enable Long-Term Islet Transplantation in Type I Diabetes
Abvance Secures Seed Funding for Safer Diabetes Management byNicole Kinsey for StartUpHealth.com, 1 July 2025.
 Abvance Therapeutics closed its initial seed financing led by Zubi Capital. The biotechnology company develops next-generation glucagon analogs and therapies to prevent hypoglycemia and improve glucose management.
Abvance Therapeutics closed its initial seed financing led by Zubi Capital. The biotechnology company develops next-generation glucagon analogs and therapies to prevent hypoglycemia and improve glucose management.
Abvance’s discovery platform builds on foundational research conducted at Vanderbilt University, including the work of Dr. Alan Cherrington, whose contributions helped shape the company’s scientific approach. Abvance is developing practical, predictable glucagon analogs and co-formulations that include glucagon analogs, designed to integrate seamlessly into modern treatment regimens for people with diabetes.
Edward Raskin has been appointed Chief Executive Officer, bringing extensive experience in diabetes-focused biotechnology and health technology. Mr. Raskin was previously a co-founder of Beta Bionics and played a key role in advancing the first bihormonal bionic pancreas. Dr. David Maggs, MD, a co-founder of Abvance, will serve as Chief Medical Officer.
Abvance’s glucagon analogs are engineered for flexible use, whether in fixed-ratio combinations with insulin taken at mealtime or in other novel applications. The Company is focused on products with pharmacologic profiles tailored to mitigate hypoglycemia without disrupting the underlying diabetes therapy. Its initial focus is on insulin-requiring populations, for whom hypoglycemia remains a persistent barrier to achieving tight glycemic control and maintaining quality of life.
Read more:
Glucotrack explains 3-year CBGM implant technology by Sean Whooley for DrugDeliveryBusiness.com, 22 July 2025.
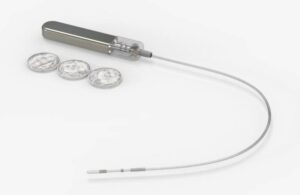 Rutherford, New Jersey-based Glucotrack completed the first-in-human study of the system. It anticipates receiving an FDA Investigational Device Exemption (IDE) in the fourth quarter of 2025. “We really want to redefine continuous glucose monitoring with the implantable long-term system,” Tapsak declared.
Rutherford, New Jersey-based Glucotrack completed the first-in-human study of the system. It anticipates receiving an FDA Investigational Device Exemption (IDE) in the fourth quarter of 2025. “We really want to redefine continuous glucose monitoring with the implantable long-term system,” Tapsak declared.
Glucotrack’s device features no external on-body components. The company designed it for three years of continuous, accurate blood glucose monitoring, providing a more convenient and less intrusive solution. Unlike traditional CGMs that measure glucose in interstitial fluid, the CBGM measures glucose levels directly from the blood. It aims to provide real-time readings without the lag time typically associated with interstitial glucose measurements.
The implant goes five centimeters within the subclavian vein. Glucotrack’s active implantable device features a small battery and electronics that are implanted just under the skin in the pectoral region. Chief Scientific Officer, Mark Tapsak explained that the implant’s location is not in a major vessel, but it can measure real-time glucose levels as pulsatile blood flows over the sensor’s tip. About the size of a USB drive, the device weighs just 6.5 grams.
According to Tapsak, Glucotrack’s device addresses specific challenges associated with working in the interstitial space, including lag times in readings, compression lows, and more. “It’s different,” Tapsak said of the company’s implant location. “It’s a protected space.”
Read more: Glucotrack explains 3-year CBGM implant technology
Shubhanshu Shukla’s space mission could rewrite the rules of diabetes care. Here’s how by Daphne Clarance for IndiaToday.in, 2 July 2025.
 Indian astronaut Shubhanshu Shukla is currently part of a historic mission aboard the International Space Station (ISS), participating in over 60 scientific experiments as part of the Axiom Mission 4 (Ax-4). Among them is a study that could reshape our understanding and management of diabetes, not just in space, but also on Earth.
Indian astronaut Shubhanshu Shukla is currently part of a historic mission aboard the International Space Station (ISS), participating in over 60 scientific experiments as part of the Axiom Mission 4 (Ax-4). Among them is a study that could reshape our understanding and management of diabetes, not just in space, but also on Earth.
A significant highlight of the Ax-4 mission is a research project called Suite Ride, designed to study how glucose metabolism is affected by microgravity. The ultimate goal is to make it safe for people with diabetes to live and work in space.
“Suite Ride is investigating how the space environment affects glucose metabolism in the human body. This can enhance our understanding of diabetes and other metabolic diseases that impact blood sugar regulation,” Dr. Mohammad Fityan, Clinical Lead for the Suite Ride project (Ax-4) from Burjeel Holdings, Chief Medical Officer, Burjeel Medical City, said. Burjeel Holdings, the research collaborator behind the project, partnered with Axiom Space to explore the performance of Continuous Glucose Monitors (CGMs) and insulin in space.
 “Microgravity allows us to study metabolism without the influence of gravity. Changes in muscle mass, fluid distribution, and circadian rhythm offer new insights into glucose metabolism and insulin sensitivity. This may lead to the identification of novel pathways and early biomarkers for insulin resistance,” said Dr. Fityan.
“Microgravity allows us to study metabolism without the influence of gravity. Changes in muscle mass, fluid distribution, and circadian rhythm offer new insights into glucose metabolism and insulin sensitivity. This may lead to the identification of novel pathways and early biomarkers for insulin resistance,” said Dr. Fityan.
“One or more astronauts are wearing CGMs for the entire duration of the mission. Readings are continuously monitored, and data is being collected. Insulin pens were sent on the flight, but astronauts are not using them. We are testing the viability and stability of insulin in the space environment. Point-of-care blood samples are also being taken during the mission to validate glucose levels.
Read more: Shubhanshu Shukla’s space mission could rewrite the rules of diabetes care
Practical Considerations and Implementation of Automated Insulin Delivery Systems by Laurel H. Messer, et al and published in the Journal of Diabetes Science and Technology, 1 July 2025.
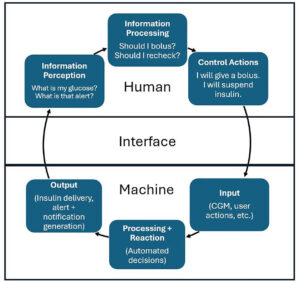 The best automated insulin delivery (AID) system is one that an individual can choose and use on a daily basis. While simple in principle, the implementation challenges are complex.
The best automated insulin delivery (AID) system is one that an individual can choose and use on a daily basis. While simple in principle, the implementation challenges are complex.
Automated insulin delivery technology has necessarily evolved with consideration of how people with diabetes (PWD) and HCPs will use the device. The ideal AID system would not require user input beyond placement and removal. As we work toward this goal, broader concepts of how humans interact with machines are adapted for the AID paradigm.
Read more: Practical Considerations and Implementation of Automated Insulin Delivery Systems
FDA Interoperability Designation—Creating Options for People With Diabetes and Pump Companies: Regulatory, Technological, and Commercial Perspectives by David C. Klonoff et al and published in the Journal of Diabetes Science and Technology, 10 September 2024.
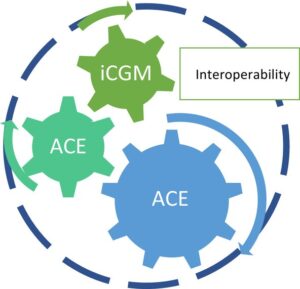 Commercial interoperable automated insulin delivery (AID) systems are currently limited to specific combinations of Alternate Controller Enabled” (ACE) pumps, interoperable continuous glucose monitors (iCGMs), and interoperable automated glycemia controllers (iAGCs). No pump manufacturer currently markets an interoperable AID system that offers the option to use more than one iAGC.
Commercial interoperable automated insulin delivery (AID) systems are currently limited to specific combinations of Alternate Controller Enabled” (ACE) pumps, interoperable continuous glucose monitors (iCGMs), and interoperable automated glycemia controllers (iAGCs). No pump manufacturer currently markets an interoperable AID system that offers the option to use more than one iAGC.
In the future, pump users will likely become increasingly knowledgeable and demanding about features of insulin delivery that can be achieved with specific iAGC algorithms. Unlocking the potential of using interoperable glycemic controllers (iAGCs) in AID systems will enable insulin pump manufacturers to provide products with greater flexibility in managing diabetes.
The Future: As AID products become increasingly established in both type 1 diabetes and type 2 diabetes, we expect two trends will affect the AID market.
-
-
-
- First, ACE pumps will exhaust the integration options with the leading iCGMs to create many AID systems, and their manufacturers will seek new, differentiating, interoperable features.
- Second, artificial intelligence will become increasingly established in iAGCs, which will increase their appeal and potential for consumerization.
-
-
We expect that both trends will lead people with diabetes to demand AID systems that contain specific ACE pumps integrated with specific iAGC software. This demand will create a commercial incentive for ACE pump companies to address the regulatory, technical, and commercial barriers to launching AID systems that integrate multiple preferred software algorithms.
Read more: FDA Interoperability Designation—Creating Options for People With Diabetes and Pump Companies




Glucotrack – seems like a great idea, however, being inside the body causes me so many questions. The most import to me is how will it communicate with a pump. If ti will not send signals to a pump it is worthless. the you have the infection issue. It seems not that great so far. But maybe someday.
3D Printing Could Enable Long-Term Islet Transplantation – would I take 3 weeks for science? Yep sign me up.
FDA Interoperability Designation – I demand interoperable software. Why not. Today we buy hardware based on speed and capabilities, but we buy software to do stuff. Why cant we shop diabetes software?