In this week’s issue of The Savvy Diabetic:
-
- Tandem Teams with Abbott Future Glucose-Ketone Sensor
- Vertex’s T1D Cure Trial Fails
- Medtronic Becomes Minimed AGAIN
- Incretins May Shield Against D Complications
- “We Don’t Know How GLP-1s Work”
- High Fasting Insulin Raises Odds for Abnormal Uterine Bleeding
- Insulet, Marvel collab to unveil comic book hero with type 1 diabetes named Omnya
- FDA Launches AI Tool to Optimize Performance
- D Stigma Among Adult T1Ds/T2Ds
- Late Dinner Bad for Metabolic Health
- Should AMA Create a Registry to Track Side Effects of GLP-1 Drugs?
- PBM Lobby Sues Arkansas over law requiring drug middlemen to sell pharmacies
Tandem, Abbott team on insulin delivery with glucose-ketone sensor by Elise Reuter for MedTechDive.com, 10 June 2025.
 Abbott announced plans in 2022 to develop a glucose-ketone sensor. The ability to detect rising ketone levels can help people with diabetes avoid a life-threatening condition called diabetic ketoacidosis, the company said in a statement. The complication occurs when the body lacks sufficient insulin to utilize blood sugar as energy, and instead begins breaking down fat for fuel, resulting in a buildup of ketones.
Abbott announced plans in 2022 to develop a glucose-ketone sensor. The ability to detect rising ketone levels can help people with diabetes avoid a life-threatening condition called diabetic ketoacidosis, the company said in a statement. The complication occurs when the body lacks sufficient insulin to utilize blood sugar as energy, and instead begins breaking down fat for fuel, resulting in a buildup of ketones.
Abbott and Tandem shared few specifics about timing or how they will integrate the new ketone sensing feature into an automated insulin delivery system. Tandem plans to integrate its available insulin delivery systems once Abbot’s new sensor is approved, Tandem Chief Strategy and Product Officer Elizabeth Gasser said.
Read more: Tandem, Abbott team on insulin delivery with glucose-ketone sensor
Critical Update: Vertex’s T1D Cure Trial Ends, Research Continues by Juvenile Diabetes Cure Alliance, thejdca.org, 1 April 2025.
 Vertex Pharmaceuticals announced that its Practical Cure project, VX-264, will not continue advancement in clinical trials after the therapy failed to achieve performance milestones at the 90-day mark. This news came at the end of March 2025, during the company’s T1D program update.
Vertex Pharmaceuticals announced that its Practical Cure project, VX-264, will not continue advancement in clinical trials after the therapy failed to achieve performance milestones at the 90-day mark. This news came at the end of March 2025, during the company’s T1D program update.
Although the end of any Practical Cure trial is disappointing, Vertex clearly reiterates its commitment to delivering a cure for T1D. Its other T1D projects, as well as a series of new ones planned, will not be affected. Vertex remains the lead horse in the race for a sustainable beta cell supply solution.
Read more: Vertex’s T1D Cure Trial Ends, Research Continues
Medtronic adopts familiar name for its planned diabetes spinout: MiniMed by Conor Hale for FierceBioTech.comk 12 June 2025.
 Medtronic has landed on a new brand for the planned spinout of its diabetes division, and it didn’t have to look very far. The new company will share a name with its flagship insulin pump, MiniMed. The moniker is also a nod to its roots, according to the company, with MiniMed being the name of the original developer of the system that Medtronic acquired for $3.7 billion back in 2001.
Medtronic has landed on a new brand for the planned spinout of its diabetes division, and it didn’t have to look very far. The new company will share a name with its flagship insulin pump, MiniMed. The moniker is also a nod to its roots, according to the company, with MiniMed being the name of the original developer of the system that Medtronic acquired for $3.7 billion back in 2001.
“Our journey began in 1983, when visionary entrepreneur Alfred E. Mann founded MiniMed and revolutionized diabetes care with many first-of-its-kind innovations that pushed the boundaries of care and helped simplify life with diabetes for countless people around the world,” said Que Dallara, current president of Medtronic’s diabetes unit and CEO-to-be of the upcoming company.
Read more: Medtronic adopts familiar name for its planned diabetes spinout: MiniMed
Beyond Blood Sugar: Could incretins shield against diabetes complications? by Tim Street for Dibettech.com, 11 June 2025.
 Beneath the daily management of blood sugar lies a far more insidious truth: high glucose isn’t just a number; it’s a silent saboteur, relentlessly chipping away at your body, cell by cell, molecule by molecule, potentially leading to devastating complications. Its long been known that keeping blood sugar in check is paramount, but the “why” behind those complications – the intricate dance of destruction at a microscopic level – and the emerging, surprising ways we can combat it, are stories often untold.
Beneath the daily management of blood sugar lies a far more insidious truth: high glucose isn’t just a number; it’s a silent saboteur, relentlessly chipping away at your body, cell by cell, molecule by molecule, potentially leading to devastating complications. Its long been known that keeping blood sugar in check is paramount, but the “why” behind those complications – the intricate dance of destruction at a microscopic level – and the emerging, surprising ways we can combat it, are stories often untold.
At the heart of this destruction lies the damage to our most vital transport system: blood vessels. High glucose levels wreak havoc on both the microscopic capillaries (microvascular complications) and the larger arteries (macrovascular complications).
But the destruction isn’t limited to the small vessels. Our major arteries – the superhighways of the circulatory system – are also under siege. High glucose, in conjunction with elevated blood fats, accelerates the relentless progression of atherosclerosis, the accumulation of sticky plaque within these arteries. This plaque narrows the arterial passages, choking off blood flow to vital organs.
The insidious nature of high glucose extends far beyond mere vascular damage, however. It orchestrates a symphony of destruction at a fundamental cellular and molecular level, initiating processes that snowball into irreversible harm. One of the chief culprits in this cellular sabotage is Advanced Glycation End Products (AGEs). It sounds complex, but it’s rather simple: think of glucose as a sticky substance. When there’s too much of it, it haphazardly binds to proteins and fats throughout your body, creating these “advanced glycation end products.” These AGEs are not inert; they are highly reactive, toxic compounds that accumulate in tissues. They stiffen blood vessel walls, stiffen and degrade nerves, and contribute to widespread inflammation and oxidative stress, acting as molecular hand grenades within your cells.
Adding to this molecular mayhem is the activation of the Polyol Pathway. In specific cells, particularly those in the eyes, kidneys, and nerves, cells that don’t require insulin to absorb glucose, unchecked high glucose levels flood their interior. This overflow forces the glucose down an alternative metabolic route known as the polyol pathway. While seemingly harmless, this pathway consumes vital protective molecules, such as NADPH, which are indispensable for neutralizing harmful free radicals. The result? A depleted antioxidant defense system leaves these vulnerable cells exposed to an onslaught of oxidative stress. In essence, the story of diabetes complications is a tale of silent cellular sabotage. But the emerging understanding of incretins offers a powerful counter-narrative. Beyond regulating blood sugar, these therapies actively engage in cellular defense, combating oxidative stress, mitigating inflammation, and reducing the damage caused by advanced glycation end-products (AGEs).
For Type 1 Diabetes, while the primary fight remains insulin replacement, the pleiotropic effects of incretins offer a fascinating adjunctive strategy, potentially providing another layer of crucial cellular protection in the ongoing battle against the silent saboteur.
Read more: Could incretins shield against diabetes complications?
After decades of research, ‘we don’t fully know’ how GLP-1s work by Erik Swain for Healio.com/endocrinology, 17 May 2025.
 Much has been learned about GLP-1s over many years, but little is understood about why the degree of response to them varies so widely, Daniel J. Drucker, MD, said in a keynote speech. Drucker, one of the first researchers to discover the benefits of GLP-1s, made his remarks at the American Association of Clinical Endocrinology’s Annual Scientific and Clinical Conference, where he received the Kahn-Tan-Faiman Frontiers in Science and Distinction in Endocrinology Award, as well as the first-ever honorary Master of the American Association of Clinical Endocrinology designation.
Much has been learned about GLP-1s over many years, but little is understood about why the degree of response to them varies so widely, Daniel J. Drucker, MD, said in a keynote speech. Drucker, one of the first researchers to discover the benefits of GLP-1s, made his remarks at the American Association of Clinical Endocrinology’s Annual Scientific and Clinical Conference, where he received the Kahn-Tan-Faiman Frontiers in Science and Distinction in Endocrinology Award, as well as the first-ever honorary Master of the American Association of Clinical Endocrinology designation.
Despite the extensive research that has gone into GLP-1s, “how does this work? The actual reality is, we don’t fully know,” Drucker said. “Long ago, we discovered that liraglutide (Victoza/Saxenda, Novo Nordisk) reduces heart attacks and strokes, and it doesn’t need to reduce blood sugar or body weight to do that in mice. We have learned that many of the GLP-1 benefits in humans are also weight-loss independent, which is fascinating.”
Read more: After decades of research, ‘we don’t fully know’ how GLP-1s work
High fasting insulin may raise odds for abnormal uterine bleeding by Michael Monstra for Healio.com/endocrinology, 10 June 2025.
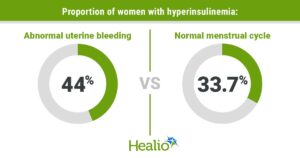 Reproductive-aged women with high fasting insulin levels could be more likely to report abnormal uterine bleeding, according to data published in Menopause.
Reproductive-aged women with high fasting insulin levels could be more likely to report abnormal uterine bleeding, according to data published in Menopause.
“While we don’t know causation at all at this point, it could be that hyperinsulinemia plays a role in the development of abnormal uterine bleeding,” Andréa C. Salcedo, DO, MPH, FACOG, assistant professor of gynecology and obstetrics at Loma Linda University School of Medicine, California, told Healio. “When providers are screening for risk factors for diabetes and cardiovascular disease in reproductive-aged women, abnormal uterine bleeding could alert the provider to evaluate their risk further. By the same token, when women begin to have dysfunctional menstrual cycles, it could also alert the provider to assess for CVD risk.”
Read more: High fasting insulin may raise odds for abnormal uterine bleeding
Insulet, Marvel collab to unveil comic book hero with type 1 diabetes by Sean Whooley for MassDevice.com, 11 June 2025.
 Insulet is bringing more visibility to the type 1 diabetes community through the launch of a comic book hero. Insulet partnered with Marvel to collaborate on a comic to make people with diabetes feel seen, understood and represented. The comic features Omnya, a high school teenager who lives with type 1 diabetes. Omnya struggles with diabetes management until she eventually embraces an insulin pump after meeting with her endocrinologist.
Insulet is bringing more visibility to the type 1 diabetes community through the launch of a comic book hero. Insulet partnered with Marvel to collaborate on a comic to make people with diabetes feel seen, understood and represented. The comic features Omnya, a high school teenager who lives with type 1 diabetes. Omnya struggles with diabetes management until she eventually embraces an insulin pump after meeting with her endocrinologist.
Readers follow Omnya’s diabetes journey as she overcomes self-doubt, discovers her power and transforms into “Dyasonic.” The Marvel-inspired hero, written by Paul Allor, inspires those with diabetes and shows how technology can lead to better outcomes and quality of life. Allor’s own experience living with diabetes informed the story, “Dyasonic: Sound of Strength.”
Read more: Insulet, Marvel collab to unveil comic book hero with T1D
FDA Launches Agency-Wide AI Tool to Optimize Performance for the American People announced by FDA.gov, 2 June 2025.
 The U.S. Food and Drug Administration (FDA) launched Elsa, a generative Artificial Intelligence (AI) tool designed to help employees—from scientific reviewers to investigators—work more efficiently. This innovative tool modernizes agency functions and leverages AI capabilities to better serve the American people.
The U.S. Food and Drug Administration (FDA) launched Elsa, a generative Artificial Intelligence (AI) tool designed to help employees—from scientific reviewers to investigators—work more efficiently. This innovative tool modernizes agency functions and leverages AI capabilities to better serve the American people.
“Today marks the dawn of the AI era at the FDA with the release of Elsa. AI is no longer a distant promise but a dynamic force enhancing and optimizing the performance and potential of every employee,” said FDA Chief AI Officer Jeremy Walsh. “As we learn how employees are using the tool, our development team will be able to add capabilities and grow with the needs of employees and the agency.”
The agency is already utilizing Elsa to expedite clinical protocol reviews, reduce the time required for scientific evaluations, and pinpoint high-priority inspection targets. Elsa is a large language model–powered AI tool designed to assist with reading, writing, and summarizing. It can summarize adverse events to support safety profile assessments, perform faster label comparisons, and generate code to help develop databases for nonclinical applications.
Read more: FDA Launches Agency-Wide AI Tool to Optimize Performance for the American People
Experiences of diabetes stigma among adults with type 1 and type 2 diabetes by Elizabeth Holmes-Truscott and others, published in Diabetic Medicine, Diabetes.org.uk, 7 June 2025.
 Diabetes stigma refers to blame, judgements, and stereotyping of people with diabetes due to their condition, which may or may not be internalised as self-stigma (shame).
Diabetes stigma refers to blame, judgements, and stereotyping of people with diabetes due to their condition, which may or may not be internalised as self-stigma (shame).
The aim of the study: To conduct a multi-study, cross-country examination of diabetes stigma among adults with type 1 and type 2 diabetes (T1D, T2D). Conclusions: Findings suggest a high and relatively consistent prevalence of diabetes stigma across studies and within and across countries, supporting calls for local and global action.
-

- International collaboration is recommended to enable monitoring and greater understanding of diabetes stigma across the world, including differential experiences of diabetes stigma.
- This study involved secondary analysis of data on diabetes stigma among adults with type 1 and type 2 diabetes from eleven original studies across six countries. Findings suggest a high and relatively consistent prevalence of diabetes stigma across studies and countries, with low levels of heterogeneity observed, supporting calls for global and local action.
- Continued international and multi-study data unification may support enhanced understanding of the occurrence, experience, and correlates of diabetes stigma, as well as protective mechanisms.
Read more: Experiences of diabetes stigma among adults with type 1 and type 2 diabetes
Late Dinner Bad for Metabolic Health Even With Later Bedtime by Crystal Phend for MedPageToday.com, 10 June 2025.
 Having a late dinner led to unhealthy metabolic patterns, according to a carefully controlled study.
Having a late dinner led to unhealthy metabolic patterns, according to a carefully controlled study.
Eating objectively late — 1 hour after rising melatonin levels mark the start of biological night — increased post-prandial glucose intolerance, with an 11% higher 4-hour glucose area under the curve and no difference in insulin compared to early dinner, 3 hours prior to the dim-light melatonin onset (DLMO).
This difference was not mitigated by delaying sleep after late dinner in the carefully controlled inpatient testing conditions of the Dinner Time 2 trial, as Daisy Duan, MD, of Johns Hopkins University School of Medicine in Baltimore, reported at the SLEEP meeting hosted jointly by the American Academy of Sleep Medicine and the Sleep Research Society.
The message is to listen to the body’s circadian clock for structuring mealtimes, commented Alexandre Abreu, MD, of the University of Miami. “After you come from a hard day of work, enjoy the time with your family, have an early meal, let the digestion process … and then finish the homework, finish the daily chores versus doing the homework, the daily chores, and then having dinner at 9, 9:30, and then go to bed.”
Read more Late Dinner Bad for Metabolic Health Even With Later Bedtime
Should AMA ‘Support’ a Registry to Track Side Effects of GLP-1 Drugs? by Shannon Firth for MedPageToday.com, 10 June 2025.
 Physicians debated the idea of the American Medical Association (AMA) supporting a registry to track adverse events associated with blockbuster diabetes and weight-loss medications during an open forum at the AMA’s annual House of Delegates meeting on Sunday.
Physicians debated the idea of the American Medical Association (AMA) supporting a registry to track adverse events associated with blockbuster diabetes and weight-loss medications during an open forum at the AMA’s annual House of Delegates meeting on Sunday.
Matthew Vo, MD, a delegate for the Organized Medical Staff Section, urged the AMA to call for the creation of a registry of GLP-1 receptor agonists such as semaglutide (Ozempic, Wegovy, Rybelsus) or the dual GLP-1/GIP agonist tirzepatide (Mounjaro, Zepbound). Vo is the author of the draft policy statement and shared his rationale for the idea, noting that advertisements characterize the medications as “miracle drugs,” but that there have been limited long-term studies on their use.
Jonathan Leffert, MD, a delegate for the American Association of Clinical Endocrinology, opposed the registry idea. Leffert said similar GLP-1 medications, such as exenatide (Byetta), have been in use since the mid-2000s as a treatment for diabetes. “We have … almost 20 years worth of experience with these drugs that are essentially life-saving drugs for our diabetics,” he stated. “We have a very good idea of what the side effects are [with] these drugs in the long-term, because they are diabetes drugs to begin with.” Leffert added that the “FDA has registries for every single drug that has ever been approved. So, there doesn’t have to be another registry.”
However, Donald Cinotti, MD, an alternate delegate for the American Academy of Ophthalmology, speaking on behalf of the Ophthalmology Section Council, pointed out that while some of the drugs have been available for 15 years, the first reports of NAION only surfaced last year. GLP-1 drugs have also been linked to an increased risk of neovascular age-related macular degeneration. “No one’s asking to use a registry to get rid of the drugs. The drugs are great. It’s about education,” Cinotti said.
Read more: Should AMA ‘Support’ a Registry to Track Side Effects of GLP-1 Drugs?
PBM lobby sues Arkansas over law requiring drug middlemen to sell pharmacies by Rebecca Pifer for HealthCareDive.com, 10 June 2025.
 Arkansas’ legislation, called Act 624, is meant to protect local community pharmacies from larger, more diversified chains by requiring companies that own both PBMs and pharmacies to shutter their operations in the state. When signing Act 624 in April, Gov. Sarah Huckabee Sanders said the law was necessary to curb anticompetitive behavior among major pharmacy benefit managers (PBMs) that threatens Arkansas’ independent pharmacies.
Arkansas’ legislation, called Act 624, is meant to protect local community pharmacies from larger, more diversified chains by requiring companies that own both PBMs and pharmacies to shutter their operations in the state. When signing Act 624 in April, Gov. Sarah Huckabee Sanders said the law was necessary to curb anticompetitive behavior among major pharmacy benefit managers (PBMs) that threatens Arkansas’ independent pharmacies.
However, PBMs — powerful middlemen that shape interactions and payments between drugmakers, payers, and pharmacies — decried the law, arguing it eliminates a significant source of efficiency in the pharmacy supply chain by forcing them to sell off their brick-and-mortar stores and halt mail-order pharmacy operations in the state. The policy would unfairly benefit Arkansas-owned pharmacies at the expense of major PBMs, they said.
Now, the PCMA, which represents 20 PBMs, including the so-called “Big Three” that control an outsized share of the market, is taking the law to court in an attempt to stop it before it takes effect at the start of 2026.
Arkansas’ law will cause roughly 40 PBM-owned pharmacies to close, along with eliminating home delivery options and jeopardizing access to specialty drugs for patients managing complex conditions, according to the PCMA’s complaint. Act 624 will also lead to job losses and reduce market competition, which could cause costs to rise for patients and their payers, the association argues.
Read more: PBM lobby sues Arkansas over law requiring drug middlemen to sell pharmacies


Depressed to hear about Vertex