In this week’s issue of The Savvy Diabetic:
- Tandem/CapBio Infusion Set Cleared (ultimately for extended use to 7 days)
- Test Strips Not Covered by Medicare
- Eversense & Sequel twiist Partnership
- Abbott Libre Lessens the Severity of CV Complications
- Call-to-Action to Eliminate Barriers to Access for AIDs
- Standby for Reviews of Upcoming CGMs & Patch Pumps, from Diabettech.com
- T1Ds Increase Daily Dose of Inhaled Insulin
- Tyrosine Kinase 2 (TYK2) Protein Reduces Pancreatic Inflammation
- How Much Sugar In Popular Breakfast Foods
- Sleep Needs & Your Nationality
Tandem Diabetes Care subsidiary earns new FDA clearance for insulin infusion set by Sean Whooley for DrugDeliveryBusiness.com, 13 May 2025.
 Tandem Diabetes Care shared in an SEC filing that its Capillary Biomedical (CapBio) subsidiary won FDA 510(k) clearance for a new infusion set. The company won clearance for its SteadiSet Infusion Set, according to an SEC Form 8-K. It’s a wearable infusion set that delivers insulin from an insulin pump to the body.
Tandem Diabetes Care shared in an SEC filing that its Capillary Biomedical (CapBio) subsidiary won FDA 510(k) clearance for a new infusion set. The company won clearance for its SteadiSet Infusion Set, according to an SEC Form 8-K. It’s a wearable infusion set that delivers insulin from an insulin pump to the body.
SteadiSet features an integrated inserter with a hidden needle designed for one-handed insertion. It received clearance to deliver insulin for up to three days of use. According to the FDA’s database, it submitted for clearance in September 2024 and received clearance last week (May 9). Tandem’s filing says CapBio designed it to provide adult patients with type 1 diabetes with continuous insulin delivery during the indicated use period.
Through CapBio, Tandem also plans to submit a separate 510(k) submission to the FDA to extend the indicated use time to up to seven days. It doesn’t expect to begin commercial activities for SteadiSet until after it receives clearance for the extended use time.
Read more: Tandem Diabetes Care subsidiary earns new FDA clearance for insulin infusion set
“Buy-Buy” Test Strips for CGM Users by Douglas L. James for IDS Diabetes In the News, IntegratedDiabetes.com, 12 May 2025.
 Starting January 1, 2025, CMS (the organization that administers Medicare and Medicaid) ruled that all users of FDA-approved CGM devices classified as “non-adjunctive” (not requiring fingerstick confirmation) were not eligible for coverage of fingerstick BG monitors and test strips. There are articles published in February 2025 by medical doctors stating the need for occasional fingersticks. This ruling will most likely be promulgated for all health plans.
Starting January 1, 2025, CMS (the organization that administers Medicare and Medicaid) ruled that all users of FDA-approved CGM devices classified as “non-adjunctive” (not requiring fingerstick confirmation) were not eligible for coverage of fingerstick BG monitors and test strips. There are articles published in February 2025 by medical doctors stating the need for occasional fingersticks. This ruling will most likely be promulgated for all health plans.
This CMS ruling did not contemplate that FDA-approved non-adjunctive CGMs all have an approval DECISION SUMMARY document that contains statements on limitations for non-adjunctive CGM use. A non-adjunctive CGM is intended to replace fingerstick BG testing for diabetes treatment decisions (using insulin or carbohydrates) except under situations when fingerstick is required to confirm CGM sensor reading (adjunctive use); otherwise, inappropriate treatment decisions may result in increased risk of severe dysglycemia when blood glucose values are acutely low or high.
Who this ruling affects: These insulin-dependent PWD are ‘in the wild’, making treatment decisions. Of PWD, most affected are the 12% dependent T1 (includes other specific types) users of bolus insulin, plus a portion of T2 with worrisome hypo events. T1s with well-controlled A1C near or within the normal range, having BG falling into the low 70s, are far less likely to be detected by a CGM unless it is fingerstick calibrated to about ±10 mg/dL. T1 PWD with an active lifestyle are handicapped by the fear of rapidly falling glucose values.
What can be done about it: Consider how much savings and lifestyle benefits from the projected costs of billions of dollars per year in the next few years, via optimally lowering A1C while avoiding hypoglycemic events, are in reach. Please immediately contact your Congressman and both Senators to withdraw the policy issue, cancel, correct, revise, or burn this CMS ruling 1738-r to restore BGM coverage and test strips when using a non-adjunctive CGM for out-of-hospital settings, so we can rest easier at 03:00 AM.
Watch for an advocacy campaign by DPAC (Diabetes Patient Advocacy Coalition) … if they develop a petition, I’ll share it here. If you are impatient to take action, please contact DPAC directly and ask them to get involved! Contact DPAC for ADVOCACY CAMPAIGN
Read more: “Buy-Buy” Test Strips for CGM Users
Eversense 365 + twiist: exploring the newest CGM/pump combo by Stacey Simms for Diabetes-Connections.com, 13 May 2025.
 This week on Diabetes Connections, the Eversense CGM gets its first pump partner. This is the implantable CGM sensor – it now lasts for a year.. and it will soon connect with the twist.. a brand new insulin pump. Join Stacey as she talks with Brian Hansen, the president of CGM at Ascenia, the company that distributes Eversense, to discuss how this will work, what’s changed for Eversense, besides the much longer wear, and what the future holds. This was a wide-ranging conversation and a fun one.
This week on Diabetes Connections, the Eversense CGM gets its first pump partner. This is the implantable CGM sensor – it now lasts for a year.. and it will soon connect with the twist.. a brand new insulin pump. Join Stacey as she talks with Brian Hansen, the president of CGM at Ascenia, the company that distributes Eversense, to discuss how this will work, what’s changed for Eversense, besides the much longer wear, and what the future holds. This was a wide-ranging conversation and a fun one.
Read more: Eversense 365 + twiist: exploring the newest CGM/pump combo
Abbott’s Libre® Technology is First Continuous Glucose Monitor Associated with Reduced Hospitalizations for Heart Complications in People with Diabetes by Abbott for PRNewsWire.com, 15 May 2025.
 Abbott announced results from its REFLECT real-world studies that show the use of FreeStyle Libre continuous glucose monitoring (CGM) technology is associated with a significant reduction in the risk of hospitalization for heart complications in people with diabetes. For the first time, data show that CGM technology can help lessen the severity of cardiovascular complications, regardless of a prior history of low blood sugar events or heart disease-related hospitalizations, in individuals with Type 1 diabetes. Findings from the studies also show a similar reduction in heart-related hospitalizations for those with Type 2 diabetes on insulin using Libre biowearable technology.
Abbott announced results from its REFLECT real-world studies that show the use of FreeStyle Libre continuous glucose monitoring (CGM) technology is associated with a significant reduction in the risk of hospitalization for heart complications in people with diabetes. For the first time, data show that CGM technology can help lessen the severity of cardiovascular complications, regardless of a prior history of low blood sugar events or heart disease-related hospitalizations, in individuals with Type 1 diabetes. Findings from the studies also show a similar reduction in heart-related hospitalizations for those with Type 2 diabetes on insulin using Libre biowearable technology.
For people with diabetes, the REFLECT findings suggest that the use of Libre technology could also potentially lead to lower healthcare costs due to the reduction in hospital admissions related to heart complications.
“These results are remarkable, as we see dual benefits from CGM technology in managing diabetes and its associated cardiovascular complications,” said one of the lead authors of the studies, David Nathanson, MD, PhD, Karolinska University Hospital in Sweden. “CGMs empower people to proactively manage their diabetes and make informed health choices through real-time, constant feedback on their glucose levels. This data shows that using CGMs is linked with significantly reduced hospitalizations related to heart issues, which can have a significant impact on patients, their families, and the healthcare system by easing medical, emotional, and financial burdens.”
The findings also reveal that the risk of hospitalizations for cardiovascular disease was reduced by 80% among people with Type 1 diabetes with no prior history of cardiovascular disease when using the Libre biowearable technology compared to those who used a traditional blood glucose monitor. For individuals with a previous history of cardiovascular disease, the risk of hospitalization was reduced by 49%.
A Call-to-Action to Eliminate Barriers to Accessing Automated Insulin Delivery Systems for People with Type 1 Diabetes was published by Diabetes Technology & Therapeutics by LiebertPub.com, 18 March 2025.
 We are issuing this call-to-action to the diabetes community (health care providers [HCPs], people with type 1 diabetes [PwT1D] and their caregivers, and payers) to fully recognize and implement the advances made over the last 10+ years in automated insulin delivery (AID) systems. Pivotal trials and real-world data provide robust and compelling evidence for the benefits of using AID systems, going beyond those of continuous glucose monitors (CGM) when added to a regimen of multiple daily insulin (MDI) injections and/or conventional pump therapy (with or without concomitant CGM).
We are issuing this call-to-action to the diabetes community (health care providers [HCPs], people with type 1 diabetes [PwT1D] and their caregivers, and payers) to fully recognize and implement the advances made over the last 10+ years in automated insulin delivery (AID) systems. Pivotal trials and real-world data provide robust and compelling evidence for the benefits of using AID systems, going beyond those of continuous glucose monitors (CGM) when added to a regimen of multiple daily insulin (MDI) injections and/or conventional pump therapy (with or without concomitant CGM).Call-to-Action: We recommend that the following model language be incorporated into current and future guidelines promulgated by professional societies and government agencies. The standard of care for glycemic management in youth and adults with T1D is an AID system. Accordingly:
- All PwT1D and other patients with insulin-dependent diabetes must be given a choice to use an AID system at the time of diagnosis or as soon after diagnosis as possible.
- The reason(s) for not giving a choice of using an AID system should be documented in the medical record.
- The choice of device should be made based on the individual’s circumstances, preferences, and needs.
- National health care systems should prioritize the provision of unfettered access to AID systems to democratize the known benefits of AID systems.
Call-to-Action Individual Endorsers: Halis K. Akturk, MD, Harpreet Bajaj, MD, Tadej Battelino, MD, Katrien Benhalima, MD, Richard M. Bergenstal MD, Emanuele Bosi, MD, Antonio Ceriello, MD, Manoj Chawla, MD, Alice G. Cheng, MD, Pratik Choudhary, MD, Ignacio Conget, MD, Martin De Bock, MD, Asma Deeb, MD, Osagie Ebekozien, MD, Nancy Elbarbary, MD, Gregory Forlenza, MD, Denise Franco, MD, Daphne Gardner Tan Su-Lyn, MD, Satish Garg, MD, Ana Maria Gomez, MD, Bruno Grassi Corrales, MD, Irl B. Hirsch, MD, Alicia Jenkins, MD, Tim Jones, MD, Shashank Joshi, MD, Partha Kar, MD, Francine Kaufman, MD, Jothydev Kesavadev, MD, Tomasz Klupa, MD, Lori Laffel, MD, Warren Lee, MD, David Maahs, MD, Julia Mader, MD, Chantal Mathieu, MD, Viswanathan Mohan, MD, Sun Joon Moon, MD, Medha Munshi, MD, Kirsten Norgaard, MD, David O’Neal, MD, Bruce Perkins, MD, Sarit Polsky, MD, Jane Reusch, MD, Banshi Saboo, MD, Antonio Saleme, MD, Peter Schwarz, MD, Viral Shah, MD, Jennifer Sherr, MD, Carol Wysham, MD, Fabian Yap Kok Peng, MD, and Muhammad Yazid Jalaludin, MD.
It’s been a while… by Tim Street for Diabettech.com, 10 May 2025.

 OK, here’s a teaser about the newest entries in the CGM market … it’s coming, from Tim Street!
OK, here’s a teaser about the newest entries in the CGM market … it’s coming, from Tim Street!
Another N=1 CGM comparison: Remember all those CGMs that everyone talked about at ATTD in March? Well, I’ve got my hands on a few of them, and we’ll put them against the incumbents. We’re waiting to see if one more becomes available before initiating the experiment towards the end of May. How will the contenders fare?
The EOFlow patch pump: This contentious device, which has lost multiple lawsuits versus Insulet’s Omnipod, is available in certain parts of the globe and can be used with AndroidAPS. I have a few coming my way and will be running them with AAPS to see how they compare to that other lower-cost patch stalwart, the Medtrum Nano.
Keep your eyes peeled, and if you see me around in late May, I may well be sporting 5 or 6 CGMs and very pricked fingers…
Read more and sign up for updates: It’s been a while…
Most adults with type 1 diabetes using inhaled insulin increase daily dose over 30 weeks by Michael Monostra for Healio.com/endocrinology, 16 May 2025.
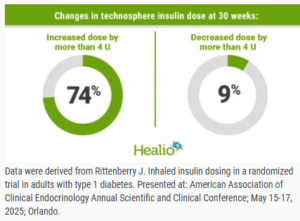 Most patients with type 1 diabetes using an inhaled insulin increased their daily insulin dose by more than 4 U from baseline to 30 weeks, according to data from the INHALE-3 trial. In the INHALE-3 extension study presented at the International Conference on Advanced Technologies & Treatments for Diabetes, significant HbA1c reductions were observed for adults who used technosphere insulin in both the main trial and extension study as well as those who were in the usual care group during the main trial and crossed over to technosphere insulin in the extension study.
Most patients with type 1 diabetes using an inhaled insulin increased their daily insulin dose by more than 4 U from baseline to 30 weeks, according to data from the INHALE-3 trial. In the INHALE-3 extension study presented at the International Conference on Advanced Technologies & Treatments for Diabetes, significant HbA1c reductions were observed for adults who used technosphere insulin in both the main trial and extension study as well as those who were in the usual care group during the main trial and crossed over to technosphere insulin in the extension study.
According to Jennifer Rittenberry, MD, medical science liaison at MannKind Corp., participants receiving technosphere insulin in INHALE-3 began the trial with a modified dose of 2 U inhaled insulin for every 1 U rapid-acting insulin they received before the trial. “[Technosphere insulin] has a unique delivery method, bioavailability, and pharmacodynamics, leading to a different clinical effect per unit compared to rapid-acting analogs,” Rittenberry said during a presentation at the American Association of Clinical Endocrinology Annual Scientific and Clinical Conference.
Read more: Most adults with type 1 diabetes using inhaled insulin increase daily dose over 30 weeks
Study reveals potential new strategy to prevent or slow progression of Type 1 diabetes by Indiana University for News-Medical.net, 7 May 2025.
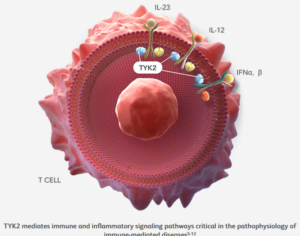 A study co-led by Indiana University School of Medicine researchers presents a potential new strategy to prevent or slow the progression of Type 1 diabetes by targeting an inflammation-related protein known to drive the disease. The findings, recently published in eBioMedicine, may help inform clinical trials of a drug that the U.S. Food and Drug Administration has already approved for psoriasis as a treatment for Type 1 diabetes.
A study co-led by Indiana University School of Medicine researchers presents a potential new strategy to prevent or slow the progression of Type 1 diabetes by targeting an inflammation-related protein known to drive the disease. The findings, recently published in eBioMedicine, may help inform clinical trials of a drug that the U.S. Food and Drug Administration has already approved for psoriasis as a treatment for Type 1 diabetes.
The researchers found that applying a molecular method to block inflammation signaling through the tyrosine kinase 2 (TYK2) protein reduced harmful inflammation in the pancreas. This strategy not only protected the beta cells in the pancreas but also reduced the immune system’s attack on those cells. A medication that inhibits TYK2 is already approved for the treatment of psoriasis, an autoimmune condition that causes skin inflammation.
Read more: Study reveals potential new strategy to prevent or slow progression of Type 1 diabetes
Which breakfast is highest in sugar? by Anahad O’Connor & Aaron Steckelberg for WashingtonPost.com, 14 May 2025.
 Is breakfast the most important meal of the day? When it comes to how much sugar you’re consuming, it just might be. Some of the most popular packaged breakfast foods — such as cereals, granolas, flavored yogurts, coffee drinks, and instant oatmeals — can harbor surprisingly large amounts of sugar.
Is breakfast the most important meal of the day? When it comes to how much sugar you’re consuming, it just might be. Some of the most popular packaged breakfast foods — such as cereals, granolas, flavored yogurts, coffee drinks, and instant oatmeals — can harbor surprisingly large amounts of sugar.
Can you guess how much sugar is lurking in some of the most popular breakfast foods and beverages? Take our quiz to test your knowledge.
Take the Quiz: Which breakfast is highest in sugar?
Here’s what your nationality says about your sleep needs by Hatty Willmoth for ScienceFocus.com, 13 May 2025.
 It doesn’t matter whether you get eight hours of sleep or six and a half – as long as that’s normal for where you live. At least, that’s according to a new study by researchers at the University of Victoria and the University of British Columbia, in Canada. The paper argues that cultural norms shape your sleep needs, so recommendations should be tailored to each country, rather than following a universal eight-hour rule.
It doesn’t matter whether you get eight hours of sleep or six and a half – as long as that’s normal for where you live. At least, that’s according to a new study by researchers at the University of Victoria and the University of British Columbia, in Canada. The paper argues that cultural norms shape your sleep needs, so recommendations should be tailored to each country, rather than following a universal eight-hour rule.
 “Sleep is shaped by more than just biology – it’s influenced by culture, work schedules, climate, light exposure, social norms and other factors,” lead author Dr Christine Ou, an assistant professor at Victoria’s School of Nursing, told BBC Science Focus. “What’s considered ‘enough’ sleep in one country might feel too much or not enough in another.”
“Sleep is shaped by more than just biology – it’s influenced by culture, work schedules, climate, light exposure, social norms and other factors,” lead author Dr Christine Ou, an assistant professor at Victoria’s School of Nursing, told BBC Science Focus. “What’s considered ‘enough’ sleep in one country might feel too much or not enough in another.”
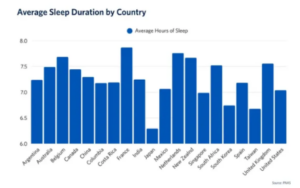
They discovered that the amount of sleep the participants were getting varied widely across the 20 countries. At the top were the French participants, who slept for an average of seven hours and 52 minutes the night before filling out the questionnaire. The shortest sleepers, meanwhile, were in Japan, where participants had slept an average of just six hours and 18 minutes.
Ou’s team also made a surprising discovery: there was no ‘ideal’ amount of sleep across all the countries that led to good health. In other words, her team found no evidence that people in countries with shorter average sleep durations had worse health as a result, compared to individuals in countries where people slept for longer each night.
“Our study found that people tend to be healthier when their sleep aligns with what’s typical in their culture,” said Ou. “Recognising cultural context can help people focus on what actually supports their health, rather than chasing a fixed number.” Within each country, the scientists found that deviating too much from the cultural sleep norm was associated with worse health outcomes, whether that meant getting too little sleep or too much.
Read more: Here’s what your nationality says about your sleep needs


wow, so much here.
I continue to say the 7 day infusion sets, lack practicality. Seven days has to be matched to 10 days, and that leaves 3 days Maybe it is just me, but either the CGMs get to 14 days, or the infusion sets should be rated as five. Lets be practical for once.
The study out of IU is such a cool step forward. Awesome. GO HOOSIERS !!! I might be partial.
This test strip issue is terrible and for the most part we have ourselves to blame. People on CGMs. should be granted 50 strips each month. Why do I say we have to blame ourselves? Look at the secondary market and you see the issue. By and large, this fight is over, and our new fight is to keep insurance coverage on non Medicare plans. Especially Medicaid. We have major work to do, but we must also clean up our own waste and abuse
I talked to someone who knows a lot about the current eversense. I am skeptical