In this week’s issue of The Savvy Diabetic:
- Arkansas Passes Law Barring PBMs From Owning Pharmacies
- NovoMix 30 and Other Brands Discontinued in India
- Medtronic Gets FDA Approvals for Interoperable Insulin Pump and Simplera Sync Glucose Sensor
- Biolinq Raises $100M for Intradermal Glucose Sensor
- Immune Protein Deficiency May Be Key to T1D Development
- Replacement for Prior Authorization
- Georgia Southern U. Proposes Insulin Pump Redesign
- Medicare Physician Pay Plummeted
Arkansas passes law banning PBMs from owning pharmacies by Rebecca Pifer for HealthCareDive.com, 21 April 2025.
 Arkansas Governor Sarah Huckabee Sanders signed a first-of-its-kind law last week, preventing pharmacy benefit managers from owning or operating pharmacies, as states increasingly move to restrict the controversial business practices of the powerful drug middlemen.
Arkansas Governor Sarah Huckabee Sanders signed a first-of-its-kind law last week, preventing pharmacy benefit managers from owning or operating pharmacies, as states increasingly move to restrict the controversial business practices of the powerful drug middlemen.
The legislation was decried by pharmacy benefit managers (PBMs), who argue that it will impede access to pharmaceuticals in the state. Yet other states could follow suit. Bills with similar provisions were recently introduced in Vermont, Texas, and New York, according to the National Community Pharmacists Association.
Read more: Arkansas passes law banning PBMs from owning pharmacies
Novo Nordisk to phase out country’s largest insulin brand by Rupali Mukherjee for TimesofIndia.indiatimes.com, 23 April 2025.
 In a move that could shake up the diabetes market, Novo Nordisk is discontinuing the country’s largest-selling insulin, NovoMix 30, as well as other older insulin brands in India. Human Mixtard alone is a Rs 800 crore brand for Novo Nordisk in India, despite it being under price control. Besides Human Mixtard, the phase-out could impact its top brands in the Rs 5,000 crore insulin market, including Actrapid, Insulatard, Insulin Detemir, Levemir, and Xultophy — marketed mostly in the format of pre-filled disposable pen and cartridges (Penfill and FlexPen).
In a move that could shake up the diabetes market, Novo Nordisk is discontinuing the country’s largest-selling insulin, NovoMix 30, as well as other older insulin brands in India. Human Mixtard alone is a Rs 800 crore brand for Novo Nordisk in India, despite it being under price control. Besides Human Mixtard, the phase-out could impact its top brands in the Rs 5,000 crore insulin market, including Actrapid, Insulatard, Insulin Detemir, Levemir, and Xultophy — marketed mostly in the format of pre-filled disposable pen and cartridges (Penfill and FlexPen).
Medtronic seeks FDA nod for insulin pump, furthering Abbott collab by Elise Reuter for MedTechDive.com, 24 April 2025.
 Medtronic is seeking Food and Drug Administration clearance for an interoperable version of its latest insulin pump, an important step in the manufacturer’s collaboration with diabetes technology rival Abbott.
Medtronic is seeking Food and Drug Administration clearance for an interoperable version of its latest insulin pump, an important step in the manufacturer’s collaboration with diabetes technology rival Abbott.
Medtronic stated that it submitted two 510(k) applications to the FDA: one for its MiniMed 780G insulin pump as an alternative controller-enabled device, and another for its SmartGuard insulin dosing algorithm as an interoperable automated glycemic controller. The clearances would enable Medtronic’s technology to be part of an automated insulin delivery system, which can adjust insulin dosing for patients based on real-time readings from glucose monitors, utilizing components manufactured by other companies.
Read more: Medtronic seeks FDA nod for insulin pump, furthering Abbott collab
Medtronic gets FDA approval to use Simplera Sync glucose sensor with insulin pumps by Elise Reuter for MedTechDive.com, 21 April 2025.
 Medtronic has received Food and Drug Administration approval for a version of its latest glucose sensor that can pair with the company’s insulin pumps. The device, called Simplera Sync, can be used with Medtronic’s MiniMed 780G insulin pumps as part of an automated insulin delivery system.
Medtronic has received Food and Drug Administration approval for a version of its latest glucose sensor that can pair with the company’s insulin pumps. The device, called Simplera Sync, can be used with Medtronic’s MiniMed 780G insulin pumps as part of an automated insulin delivery system.
Medtronic plans to launch the sensor on a limited basis this fall. CEO Geoff Martha told investors in February that the company expects Simplera Sync, and a new glucose monitor being developed with Abbott, to grow the company’s U.S. diabetes business.
Biolinq raises $100M for intradermal glucose sensor for type 2 diabetes by Jessica Hagen for MobiHealthNews.com, 22 April 2025.
 Biolinq, a health technology company developing precision multi-analyte wearable biosensors for metabolic health, announced it secured $100 million in Series C funding. Alpha Wave Ventures led the round with participation from existing investors AXA IM Alts, M Ventures, LifeSci Venture Partners, RiverVest Venture Partners, Hikma Ventures, Taisho Pharmaceutical, Features Capital and Aphelion Capital.
Biolinq, a health technology company developing precision multi-analyte wearable biosensors for metabolic health, announced it secured $100 million in Series C funding. Alpha Wave Ventures led the round with participation from existing investors AXA IM Alts, M Ventures, LifeSci Venture Partners, RiverVest Venture Partners, Hikma Ventures, Taisho Pharmaceutical, Features Capital and Aphelion Capital.
Biolinq offers a wearable patch that evaluates glucose levels and provides insights into metabolic health for individuals with type 2 diabetes who are not on insulin. The patch uses small electrochemical sensors to measure glucose levels from the intradermal space just beneath the skin’s surface.
The California-based company’s glucose sensor is an investigational device that has not been cleared or approved by the FDA. “This financing will bolster Biolinq’s commercial readiness efforts while we pursue regulatory approval for the first intradermal glucose sensor that incorporates activity and sleep information into a single wearable device,” Rich Yang, CEO of Biolinq, said in a statement.
Immune Protein Deficiency May Be Key to Type 1 Diabetes Development from the School of Medicine at the University of Missouri and reported on medicine.missouri.edu, 18 April 2025.
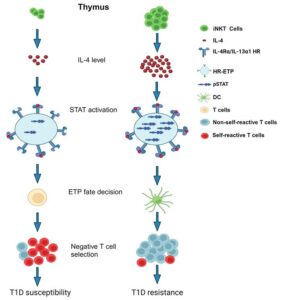 While it’s not fully understood why type 1 diabetes develops, a specific deficiency may be at play. Researchers discovered a specific protein that helps regulate immune system responses, IL-4, is low in nonobese diabetic mice.
While it’s not fully understood why type 1 diabetes develops, a specific deficiency may be at play. Researchers discovered a specific protein that helps regulate immune system responses, IL-4, is low in nonobese diabetic mice.
“We found that the cells that produce IL-4, called iNKT cells, are also low in number in nonobese diabetic mice,” study author Habib Zaghouani said. “This suggests some sort of deficiency or malfunction in the thymus gland, which is also part of the immune system and where these cells are produced.”
Without enough IL-4 protein, too few dendritic cells are produced, which are immune cells that identify threats to the body. These immune cells can also detect T cells, also called lymphocytes or white blood cells, that attack body processes like insulin production and delete them, so they don’t cause further harm.
“In type 1 diabetes, the lack of IL-4 lets these self-reactive T cells do as they please, harming vital processes our body needs to function,” Zaghouani said. Zaghouani plans to continue his research by determining why iNKT cells are low in number for individuals with type 1 diabetes. These answers could explain why type 1 diabetes develops, which would allow researchers to discover ways to prevent the disease. Zaghouani says an injection containing supplemental IL-4 protein could strengthen the immune system against type 1 diabetes. He says such a treatment would need to undergo clinical trials to ensure safety and effectiveness.
Read more: Immune Protein Deficiency May Be Key to Type 1 Diabetes Development
Here’s a Replacement for Prior Authorization by Michele Kowalski-McGraw, MD/MPH, Larry Ozeran, MD, Manijeh Berenji, MD/MPH, and Richard Schreiber, MD, for MedPageToday.com, 27 April 2025.
What happens when prescribed care is denied?
 The stated objectives of prior authorization (PA) are to reduce unnecessary care and costs. However, as a 2022 Department of Health and Human Services Office of the Inspector General report highlighted, prior authorization can reduce access to care by delaying or denying medically necessary medications and services. Several studies suggest prior authorization costs billions of dollars annually. According to an American Medical Association survey, prior authorization creates administrative roadblocks that frequently result in delayed care; increases costs for patients, caregivers, clinicians, and employers; and contributes to moral injury. Nearly 25% of physicians report that prior authorization has led to a serious adverse event for a patient in their care.
The stated objectives of prior authorization (PA) are to reduce unnecessary care and costs. However, as a 2022 Department of Health and Human Services Office of the Inspector General report highlighted, prior authorization can reduce access to care by delaying or denying medically necessary medications and services. Several studies suggest prior authorization costs billions of dollars annually. According to an American Medical Association survey, prior authorization creates administrative roadblocks that frequently result in delayed care; increases costs for patients, caregivers, clinicians, and employers; and contributes to moral injury. Nearly 25% of physicians report that prior authorization has led to a serious adverse event for a patient in their care.
Given these alarming statistics, the End Burnout Group (EBG), a multi-disciplinary group of clinicians and informaticists that we lead, identified prior authorization as a leading root cause of burnout. We propose evidence-based care optimization (ECO) to minimize unnecessary care while enhancing patient care, supporting clinicians, and benefiting the economy. ECO leverages existing digital tools to automate the certification of clinical appropriateness, utilizing national clinical guidelines, and integrates a separate digital insurance query to determine coverage terms. The goal is to separate the determination of clinical appropriateness from insurance coverage.
Health insurers use prior authorization to make clinical decisions, despite the inherent conflict of interest: the lowest-cost care is often no care. ECO shifts the current paradigm by trusting clinical judgment in care decisions while verifying that it meets clinical guidelines. This approach can end inappropriate denials and unnecessary delays that harm patients.
Read more: Here’s a Replacement for Prior Authorization
Georgia Southern researcher comes in first place with international winning proposal for insulin pump redesign by Georgia Southern University, published by GriceConnect.com, 20 April 2025.
Medicare physician pay has plummeted since 2001 byTanya Albert Henry for AMA-Assn.org, 21 April 2025.
 Medicare physician payment—often referred to as Medicare reimbursement—must be tied to an inflation index known as the Medicare Economic Index (MEI). As part of its campaign to reform the unsustainable Medicare payment system, the AMA has outlined in a concise and easily navigable manner why this payment reform is necessary now.
Medicare physician payment—often referred to as Medicare reimbursement—must be tied to an inflation index known as the Medicare Economic Index (MEI). As part of its campaign to reform the unsustainable Medicare payment system, the AMA has outlined in a concise and easily navigable manner why this payment reform is necessary now.
The significant issue is that since 1992, the role of MEI in shaping Medicare physician payment has declined substantially, first under the sustainable growth rate (SGR) and then under the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015. Given those changes, it comes as no surprise that, when adjusted for inflation, Medicare physician payments have effectively declined by 33% from 2001 to 2025.
Jason Goldman, MD, a Florida internist, said Congress needs “to fix Medicare now, not just because physicians need to be paid fairly, but because the patients need their physicians. And if they do not fix Medicare, we will not have a health care system.” The AMA is leading the charge to reform the Medicare payment system.


