In this week’s issue of The Savvy Diabetic:
- Breakthrough T1D Gratitude for Extension of Special Diabetes Program
- Funding Cut to Diabetes Prevention Program (DPP)
- Sequel Partners Integrates with Abbott FreeStyle Libre 3 Plus with Twiist Pump
- Afrezza Lowers A1C in 30 Weeks
- ATTD 2025 on Dexcom 15-day Sensor and Tandem Control-IQ for T2D
- Global Increase in Diabetic Nephropathy
- Non-Invasive Glucose Monitoring Using Photoacoustic Sensing
- Present & Future Cell Therapies for T1D
- Major Changes in New CKD Management Guidelines – KDIGO
- Medtronic InPen Device Issue
- Duodenal Mucosal Resurfacing for Metabolic Diseases
- Neural Origins of Sugar Cravings
Breakthrough T1D Expresses Gratitude for Extension of Special Diabetes Program, posted by BreakthroughT1D.org, 17 March 2025.
 Breakthrough T1D, formerly JDRF, is grateful that Congress and the President enacted a six-month, $80 million extension of the Special Diabetes Program (SDP), a crucial program that contributes millions of dollars to type 1 diabetes (T1D) research annually through the National Institutes of Health.
Breakthrough T1D, formerly JDRF, is grateful that Congress and the President enacted a six-month, $80 million extension of the Special Diabetes Program (SDP), a crucial program that contributes millions of dollars to type 1 diabetes (T1D) research annually through the National Institutes of Health.
“We deeply appreciate our Breakthrough T1D champions who have continued to support and push for renewal of the Special Diabetes Program and the critical funding it provides for type 1 diabetes research,” said Breakthrough T1D Chief Global Advocacy Officer Lynn Starr. “We’d especially like to thank Senate Diabetes Caucus co-chairs, Senators Susan Collins (R-ME) and Jeanne Shaheen (D-NH), and Congressional Diabetes Caucus co-chairs, Representatives Diana DeGette (D-CO) and Gus Bilirakis (R-FL), for their leadership and commitment to improving the lives of Americans affected by type 1 diabetes.”
Read more: Breakthrough T1D Expresses Gratitude for Extension of Special Diabetes Program
Top Societies Decry Trump’s Funding Cuts to Landmark Diabetes Study by Kristen Monaco for Medpage Today.com, 20 March 2025.
 The Endocrine Society and American Diabetes Association criticized the cancellation of funding for the ongoing, landmark Diabetes Prevention Program (DPP), which has been tracking people with diabetes and prediabetes for 30 years. Researchers working on the study, which kicked off in 1996, found out last week that the study’s NIH funding was yanked by the Trump administration. DPP investigators were told to immediately stop study activities.
The Endocrine Society and American Diabetes Association criticized the cancellation of funding for the ongoing, landmark Diabetes Prevention Program (DPP), which has been tracking people with diabetes and prediabetes for 30 years. Researchers working on the study, which kicked off in 1996, found out last week that the study’s NIH funding was yanked by the Trump administration. DPP investigators were told to immediately stop study activities.
“The research provides an important source of long-term information on diabetes prevention,” the Endocrine Society said in a statement. “The Society is concerned about how the loss of this ongoing research, which is being conducted at 30 institutions in 21 states, will impact tens of millions of people who have diabetes and prediabetes nationwide.”
Direct and indirect costs of treating diagnosed cases of diabetes nationwide totaled around $413 billion in 2022. “Preventing and delaying the onset of diabetes can help reduce other chronic conditions, such as heart and kidney disease, and control healthcare costs,” the Endocrine Society pointed out. “Eliminating the Diabetes Prevention Program contradicts the country’s commitment to addressing chronic disease and making America healthy.”
The American Diabetes Association said it was “extremely concerned about the impact” of the canceled funding and is “engaging with congressional leaders on diabetes and the Trump administration to express our concerns, especially as this funding decision seems to be at odds with the Department of Health and Human Services’ commitment to combatting chronic disease in the United States.”
Read more: Top Societies Decry Trump’s Funding Cuts to Landmark Diabetes Study
Sequel Med Tech makes First CGM integration with Abbott for automated insulin pump by Sean Whooley for DrugDeliveryBusiness.com, 18 March 2025.
 Sequel Med Tech announced today that it integrated its twiist automated insulin delivery (AID) with the Abbott FreeStyle Libre 3 Plus sensor. This marks the first continuous glucose monitor (CGM) integration for twiist. Manchester, New Hampshire-based Sequel expects to launch the combination in the second quarter of 2025. “We are thrilled to announce our collaboration with Abbott, integrating its world-leading FreeStyle Libre technology as our first CGM partner, marking a significant milestone as we bring our innovative AID system to market,” said Dr. Alan Lotvin, CEO of Sequel.
Sequel Med Tech announced today that it integrated its twiist automated insulin delivery (AID) with the Abbott FreeStyle Libre 3 Plus sensor. This marks the first continuous glucose monitor (CGM) integration for twiist. Manchester, New Hampshire-based Sequel expects to launch the combination in the second quarter of 2025. “We are thrilled to announce our collaboration with Abbott, integrating its world-leading FreeStyle Libre technology as our first CGM partner, marking a significant milestone as we bring our innovative AID system to market,” said Dr. Alan Lotvin, CEO of Sequel.
Read more: Sequel Med Tech makes first CGM integration with Abbott for automated insulin pump
Inhaled insulin lowers HbA1c at 30 weeks in adults with type 1 diabetes by Michael Monostra for Healio.com/endocrinology, 21 March 2025.
 Technosphere insulin, a rapid-acting human insulin administrated through an inhaler, was associated with reductions in HbA1c at 30 weeks for adults with type 1 diabetes, according to findings from the INHALE-3 extension trial. During a symposium at the International Conference on Advanced Technologies & Treatments for Diabetes, data from the INHALE-3 extension trial showed adults in the technosphere insulin group had a reduction in HbA1c from the end of the 17-week primary trial to 30 weeks. Adults in the usual care group during INHALE-3 who switched to technosphere insulin during the extension trial were also able to reduce their HbA1c.
Technosphere insulin, a rapid-acting human insulin administrated through an inhaler, was associated with reductions in HbA1c at 30 weeks for adults with type 1 diabetes, according to findings from the INHALE-3 extension trial. During a symposium at the International Conference on Advanced Technologies & Treatments for Diabetes, data from the INHALE-3 extension trial showed adults in the technosphere insulin group had a reduction in HbA1c from the end of the 17-week primary trial to 30 weeks. Adults in the usual care group during INHALE-3 who switched to technosphere insulin during the extension trial were also able to reduce their HbA1c.
The findings revealed that technosphere insulin could be an important tool in glycemic management for some adults with type 1 diabetes, though patients must be vigilant about the timing of administration, according to Irl Hirsch, MD, professor of medicine in the division of metabolism, endocrinology and nutrition at the University of Washington School of Medicine and an investigator on the INHALE-3 trial.
“Using technosphere insulin may be good for patients, [but] they have to be engaged in their diabetes self-management, [and] want to reduce their hyperglycemia even further,” Hirsch said during the presentation. “UDexcomsing technosphere insulin may be good for patients who want an alternative to an [insulin] pump. Not everybody wants a pump, we have a lot of our patients who didn’t want to carry it, and they are still on technosphere insulin to this day.”
Read more: Inhaled insulin lowers HbA1c at 30 weeks in adults with type 1 diabetes
Dexcom shares 15-day CGM data; Tandem posts Type 2 pivotal data by Elise Reuter for MedTechDive.com, 20 March 2025.
 Dexcom shared the first data for its 15-day continuous glucose monitor at ATTD Amsterdam. The new device would give Dexcom a CGM that can be worn for as long as competitor Abbott’s Freestyle Libre 3 Plus sensor. COO Jake Leach said in a February earnings call that the company hopes to receive Food and Drug Administration clearance in the second half of the year. Dexcom said the new sensor has a mean absolute relative difference of 8%, making it the most accurate CGM. MARD measures the average difference between device measurements and reference measurements, such as fingerstick tests.
Dexcom shared the first data for its 15-day continuous glucose monitor at ATTD Amsterdam. The new device would give Dexcom a CGM that can be worn for as long as competitor Abbott’s Freestyle Libre 3 Plus sensor. COO Jake Leach said in a February earnings call that the company hopes to receive Food and Drug Administration clearance in the second half of the year. Dexcom said the new sensor has a mean absolute relative difference of 8%, making it the most accurate CGM. MARD measures the average difference between device measurements and reference measurements, such as fingerstick tests.
 Tandem Diabetes Care shared new data on Wednesday that supported FDA clearance of its Control-IQ+ algorithm for Type 2 diabetes. The clearance made Tandem the second company with an authorized automated insulin delivery system, which integrates data from glucose monitors with insulin pumps, after competitor Insulet received an expanded indication in August. Tandem found that people who used its Control-IQ+ algorithm paired with Dexcom’s G6 sensor had a 0.9% reduction in A1C compared with a 0.3% reduction in the control group, who used the sensor paired with whatever insulin delivery method they used prior to the study. The trial also found that users’ time spent in a target blood sugar range improved by 16% with Control-IQ+, an increase of 3.4 hours per day compared to the control group. The results were published in the New England Journal of Medicine.
Tandem Diabetes Care shared new data on Wednesday that supported FDA clearance of its Control-IQ+ algorithm for Type 2 diabetes. The clearance made Tandem the second company with an authorized automated insulin delivery system, which integrates data from glucose monitors with insulin pumps, after competitor Insulet received an expanded indication in August. Tandem found that people who used its Control-IQ+ algorithm paired with Dexcom’s G6 sensor had a 0.9% reduction in A1C compared with a 0.3% reduction in the control group, who used the sensor paired with whatever insulin delivery method they used prior to the study. The trial also found that users’ time spent in a target blood sugar range improved by 16% with Control-IQ+, an increase of 3.4 hours per day compared to the control group. The results were published in the New England Journal of Medicine.
Read more:
1990 to 2021 Saw Increase in Global Burden of Diabetic Nephropathy by Elana Gotkine for Healthday.com, 17 March 2025.
 The global burden of diabetic nephropathy increased from 1990 to 2021, and is projected to continue increasing to 2050, according to a study published online Feb. 21 in Frontiers in Endocrinology. Xiao Ma, from The Third People’s Hospital of Chengdu in China, and colleagues used data from 1990 to 2021 to analyze the trends and future projections of the worldwide burden of chronic kidney disease (CKD) caused by type 1 and type 2 diabetes mellitus based on the Global Burden of Disease Study.
The global burden of diabetic nephropathy increased from 1990 to 2021, and is projected to continue increasing to 2050, according to a study published online Feb. 21 in Frontiers in Endocrinology. Xiao Ma, from The Third People’s Hospital of Chengdu in China, and colleagues used data from 1990 to 2021 to analyze the trends and future projections of the worldwide burden of chronic kidney disease (CKD) caused by type 1 and type 2 diabetes mellitus based on the Global Burden of Disease Study.
“In the absence of interventions, the global burden of diabetic nephropathy is projected to continue to rise annually from 2022 to 2050, placing even greater stress on the global health system in the future,” the authors write.
Read more: 1990 to 2021 Increase in Global Burden of Diabetic Nephropathy
No more finger pricks? IISc successfully uses light and sound to make diabetes tests painless by Chetana Belagere for SouthFirst.com/health, 20 March 2025.
 Scientists at the Indian Institute of Science (IISc) Bengaluru have developed a painless and needle-free diabetes test that relies on light and sound rather than invasive blood sampling. The new method, using photoacoustic sensing, uses laser beams to measure glucose concentration inside the body without breaking the skin.
Scientists at the Indian Institute of Science (IISc) Bengaluru have developed a painless and needle-free diabetes test that relies on light and sound rather than invasive blood sampling. The new method, using photoacoustic sensing, uses laser beams to measure glucose concentration inside the body without breaking the skin.
“When a laser shines on biological tissue, the light is absorbed, causing the tissue to heat up slightly and expand. This expansion creates tiny vibrations that generate sound waves,” explained Jaya Prakash, Assistant Professor at the Department of Instrumentation and Applied Physics (IAP) at IISc and corresponding author of the study published in Science Advances.
The researchers, according to a release by the IISc says that they discovered that glucose changes the intensity of these sound waves, allowing them to measure blood sugar levels accurately. The IISc team’s innovation could be a game-changer by eliminating the need for any kind of penetration.
Despite its promise, the technology is not yet ready for commercial use. One of the biggest challenges is the size and cost of the laser setup. “The laser source we use right now generates very small nanosecond pulses, which makes it expensive and bulky. We need to make it more compact and affordable to bring it into clinical practice,” said Padmanabhan.
Read more:
The present and future of cell therapies for T1D by BreakthroughT1D.org, 17 March 2025.
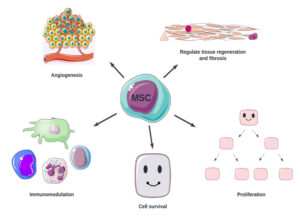 We’ve made major progress in developing cell replacement therapies for type 1 diabetes (T1D) over the past couple of decades. However, there is more work to do, to ensure the cells survive and function, ideally without immunosuppression, and ensure these therapies are accessible to everyone with T1D.
We’ve made major progress in developing cell replacement therapies for type 1 diabetes (T1D) over the past couple of decades. However, there is more work to do, to ensure the cells survive and function, ideally without immunosuppression, and ensure these therapies are accessible to everyone with T1D.
-
- Priority #1: Cell source: To meet the challenge of not enough insulin-producing cells, the solution is to scale up the production of manufactured islets. Breakthrough T1D is funding an initiative at the Advanced Regenerative Manufacturing Institute (ARMI) to scale up Dr. Jeffrey Millman’s protocol to generate unlimited manufactured islets in a reliable, automated, and reproducible way. Breakthrough T1D is also building a partnership with the Cedars-Sinai Biomanufacturing Center to accomplish this goal.
- Priority #2: Cell survival: To meet the challenge of where and how manufactured islets are implanted determines whether they survive, the solution is to figure out the best place for implanted islets to live in the body. Breakthrough T1D is funding research to develop scaffolds, which are specialized biomaterials that islet cells can stick to and get nutrients and oxygen to help them survive. Similarly, islets implanted in encapsulation devices, such as Sernova’s Cell Pouch, can access nutrients and oxygen with the added benefit of being protected from the immune system. There are also various Breakthrough T1D-funded clinical studies that are investigating different places in the body for manufactured islets, including the omentum and abdominal wall.
- Priority #3: Cell Protection: To meet the challenge that implanted islets will be attacked by the immune system if not for immunosuppressants, which come with problems of their own, the solution is to find a better way to protect transplanted islets from the immune system. Breakthrough T1D is funding research and clinical trials, including genetically engineered islets that can evade immune detection, encapsulation devices, and immunomodulatory therapeutics that can dampen the immune response.
Read more; The present and future of cell therapies for T1D
Major Changes in New CKD Management Guidelines by Marilynn Larkin for Medscape.com, 19 March 2025.
 Updates on chronic kidney disease (CKD) diagnosis, use of sodium-glucose cotransporter-2 (SGLT-2) inhibitors as first-line therapy, and personalized treatment approaches for kidney and cardiovascular risk reduction are among the new CKD management recommendations from the Kidney Disease: Improving Global Outcomes (KDIGO) organization.
Updates on chronic kidney disease (CKD) diagnosis, use of sodium-glucose cotransporter-2 (SGLT-2) inhibitors as first-line therapy, and personalized treatment approaches for kidney and cardiovascular risk reduction are among the new CKD management recommendations from the Kidney Disease: Improving Global Outcomes (KDIGO) organization.
“The last CKD guideline was published in 2012, and there have been so many exciting contributions to nephrology in the past years that the guideline required an update,” Magdalena Madero, MD, of the National Heart Institute in Mexico City, Mexico, told Medscape Medical News. “SGLT-2 inhibitors, glucagon-like peptide-1 (GLP-1) agonists, and nonsteroidal mineralocorticoid receptor antagonists (ns-MRAs) are some of the new fascinating drugs that [have] modified the course of CKD.”
Madero is lead author of a recent synopsis of the KDIGO guideline, published online in the Annals of Internal Medicine, that focuses on the recommendations with the most evidence. According to Madero, the most important takeaways for clinicians are:
-
-
- Broad implementation of albuminuria and cystatin C for CKD diagnosis and risk stratification
- The use of SGLT-2 inhibitors as first-line therapy for CKD
- Stopping angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) for hyperkalemia management, and, in addition, only using nsMRAs as a last resort, “after diet/potassium binders/loop diuretics have been used.”
-
Read more: Major Changes in New CKD Management Guidelines
MEDICAL DEVICE CORRECTION: InPen™ smart insulin pen Cartridge Holder Assembly Issue was issued by Medtronic.com, March 2025.
 Medtronic is contacting you with a medical device correction regarding InPen smart insulin pen. Some InPens have been identified with an issue that can cause difficulty in removing the cartridge holder or installing the insulin cartridge into the cartridge holder.
Medtronic is contacting you with a medical device correction regarding InPen smart insulin pen. Some InPens have been identified with an issue that can cause difficulty in removing the cartridge holder or installing the insulin cartridge into the cartridge holder.
Issue Description: Some InPens from certain lots may have been incorrectly assembled. For affected InPens, users could potentially experience either one of two issues: either the insulin cartridge will not fit into the cartridge holder; or the cartridge holder may be difficult to remove from the insulin pen, requiring a significant amount of force.
Risk to Health: An InPen with an insulin cartridge that will not fit into the cartridge holder cannot be used. An InPen with a cartridge holder that is difficult to remove should not be used even if you are able to do so. If you do not have backup insulin when you need it, you may experience temporary hyperglycemia. Between May 2024 and November 2024, Medtronic received 31 complaints potentially related to this issue. There have not been any serious adverse events associated with these complaints.
If you have an InPen from one of the affected lot numbers listed at the top of this letter and have not yet started using it or have had difficulty in removing the cartridge holder or inserting the insulin cartridge, please call 1-800-646-4633, option 1, or go to https://info.medtronicdiabetes.com/ICH-replacement-form to request a replacement. Do not use this InPen and dispose of it according to local regulations.
Read more: InPen smart insulin pen Cartridge Holder Assembly Issue
Duodenal mucosal resurfacing: Treatments for metabolic diseases by Nikki Thompson for MedicalDevice-Network.com, 5 March 2025.
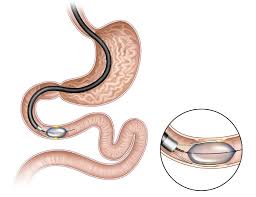 An innovative endoscopic procedure known as duodenal mucosal resurfacing (DMR) offers new hope for patients. This minimally invasive treatment targets abnormalities in the gut, offering patients a way to regulate glucose and cholesterol levels, reduce liver fat, and achieve weight loss. With its potential to improve metabolic health significantly, DMR is revolutionizing care for patients with these chronic conditions.
An innovative endoscopic procedure known as duodenal mucosal resurfacing (DMR) offers new hope for patients. This minimally invasive treatment targets abnormalities in the gut, offering patients a way to regulate glucose and cholesterol levels, reduce liver fat, and achieve weight loss. With its potential to improve metabolic health significantly, DMR is revolutionizing care for patients with these chronic conditions.
The duodenum, the first part of the small intestine, plays a crucial role in nutrient absorption and metabolic regulation. In patients with metabolic diseases, the duodenal lining often becomes dysfunctional, contributing to insulin resistance and other metabolic imbalances. Duodenal mucosal resurfacing addresses this by using an endoscopic ablation catheter to rejuvenate the mucosal lining and restore its proper function.
Patients undergoing the procedure frequently experience better glycaemic control, with reductions in HbA1c levels – a key marker of blood sugar management. Additionally, DMR has been shown to lower cholesterol levels, decrease liver fat, and support weight loss, making it a comprehensive treatment for metabolic dysfunction. These benefits extend beyond glucose control, offering a holistic improvement in patients’ overall health.
Read more: Duodenal mucosal resurfacing: Treatments for metabolic diseases
Why there’s always room for dessert, according to brain science by Emily Kwong & Andrea Muraskin for NPR.org, 15 March 2025.
 You probably know the feeling of having a hearty meal at a restaurant, and feeling full and satisfied … only to take a peek at the dessert menu and decide the cheesecake looks just irresistible. So why is it that you just absolutely couldn’t have another bite, but you somehow make an exception for a sweet treat? Scientists now have a better understanding of the neural origins of this urge thanks to a recent study published in the journal Science.
You probably know the feeling of having a hearty meal at a restaurant, and feeling full and satisfied … only to take a peek at the dessert menu and decide the cheesecake looks just irresistible. So why is it that you just absolutely couldn’t have another bite, but you somehow make an exception for a sweet treat? Scientists now have a better understanding of the neural origins of this urge thanks to a recent study published in the journal Science.
“When we taste something sweet, it’s not just the sugar we’re consuming — it’s triggering a system in the brain that associates that sweet taste with pleasure, which makes us want to keep eating,” says Dr. Paule Joseph, a researcher who studies metabolism at the National Institutes of Health and was not associated with the study. When the team then blocked this opioid pathway, the mice stayed away from the sugar.
The researchers found the same neural mechanism in humans when studying donated brain tissue and scanning the brains of volunteers, who sat in an fMRI machine and were fed a sugar solution through a tube. This led the scientists to conclude, says Fenselau, that in humans like in mice “the opiate action in this part of the brain drives that consumption of high-sugar containing foods.”
Results suggest that people’s brains evolved to love sugar in excess. Other research has found a link between sugar consumption and the dopamine system in our brain; some research even suggests sugar’s effect on the brain in the long-run can be similar to addictive drugs.
Read more: Why there’s always room for dessert, according to brain science


The CKD items are significant if they are implemented. I have no idea if they will be or how long before they are, but when?
Defunding the DPP is one of the stupidest things this administration has done. Hands down.