Novo Nordisk challenges House report it collaborated with competitors on insulin prices was written by Jonathan Block for SeekingAlpha.com, 2 February 2022.
 Novo Nordisk CEO Lars Joergensen struck back at a House Oversight Committee report issued in December alleging that the company had collaborated with rivals to raise insulin prices for years. “Our net pricing is actually declining quite significantly,” Jorgensen said today, Reuters reported. “For quite some years pricing has been going down on insulin, not going up.”
Novo Nordisk CEO Lars Joergensen struck back at a House Oversight Committee report issued in December alleging that the company had collaborated with rivals to raise insulin prices for years. “Our net pricing is actually declining quite significantly,” Jorgensen said today, Reuters reported. “For quite some years pricing has been going down on insulin, not going up.”
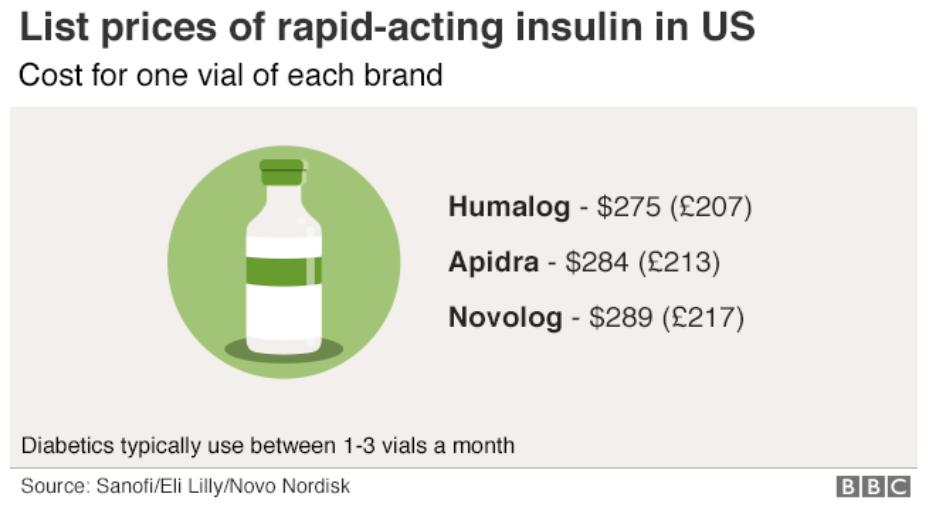
That report, issued in December, took Novo, Eli Lilly and Novartis to task as the three companies control 90% of the insulin market. “Over the past 20 years, they have repeatedly and dramatically raised the list prices of their rapid-acting and long-acting insulins and reaped billions of dollars in revenues,” the report states.
Despite Jorgensen’s assertions, the report contends Novo has raised the price of Novolog by 628% since 2001 through 28 price hikes. Those increases happened in much the same fashion as hikes for Lilly’s Humalog. In its annual report released today, Novo claimed that net prices on its insulin products in the U.S. have decreased every year since 2017. The report also stated that Novo is facing eight lawsuits over diabetes pricing. Lilly and Novartis have also been named defendants.
Read more: Novo Nordisk challenges House report it collaborated with competitors on insulin prices
Double Diabetes: Can You Have Both Type 1 and Type 2 Diabetes at the Same Time? was written by Steve Edelman, MD for TCOYD.org, 21 January 2022.
Type 1 and type 2 diabetes are two completely different diseases, but they have one thing in common – high blood sugars, if not treated properly. Certain therapies and technologies are also used to treat both type 1 and type 2.
So how does someone get lucky enough to have both type 1 and type 2 diabetes? When both of your parents have type 2, your chances of getting type 2 during your lifetime is virtually 100%. Double diabetes is not that uncommon, and it is important to have a level of awareness if you are at risk.
Read more: Double Diabetes: Can You Have Both Type 1 and Type 2 Diabetes at the Same Time?
New diabetes targets could allow protection of pancreatic beta cells was reported by Children’s Hospital Boston, 3 February 2022.
 A central feature of type 1 diabetes is loss of the pancreatic beta cells that make insulin. Researchers led by Paolo Fiorina, MD, Ph.D., of Boston Children’s Hospital and Francesca D’Addio, MD, of the University of Milan now identify a harmful cellular pathway that causes these cells to die. When they blocked the pathway in mice and in human islets, where beta cells reside, they preserved beta cells, increased insulin production, and prevented or delayed the onset of diabetes.
A central feature of type 1 diabetes is loss of the pancreatic beta cells that make insulin. Researchers led by Paolo Fiorina, MD, Ph.D., of Boston Children’s Hospital and Francesca D’Addio, MD, of the University of Milan now identify a harmful cellular pathway that causes these cells to die. When they blocked the pathway in mice and in human islets, where beta cells reside, they preserved beta cells, increased insulin production, and prevented or delayed the onset of diabetes.
The study, published February 3rd in Nature Communications, used three different mouse models of diabetes and found protective effects whether the pathway was blocked genetically or with an antibody. Studies of human cells and people with diabetes were consistent with the mouse findings. The pathway, they showed, consists of a “death receptor” on insulin-producing beta cells called TMEM219, coupled with insulin-like growth factor binding protein 3 (IGFBP3), which interacts with this receptor. When IGFBP3 binds to TMEM219, the researchers found, beta cells die through the process of apoptosis.
“We believe this might be a natural mechanism to keep the beta cell population under control,” says Fiorina. “We think that in disease, IGFBP3 production may be increased, so there is a loss of beta cells. The common thought for type 1 diabetes is that it autoimmune.But immunotherapy doesn’t completely cure diabetes. We think that dysregulation of beta cell homeostasis also plays a role and that IGFBP3 acts as a ‘betatoxin.'”
Fiorina founded a biotechnology company in Italy called Enthera in 2016 that is developing biologics based on these discoveries. First-in-human tests of an antibody treatment to block the IGFBP3/TMEM219 pathway could begin as early as September 2022 in Europe.
Read more: New diabetes targets could allow protection of pancreatic beta cells
Bright indoor lighting during the day may lower glucose, improve energy expenditure was reported by Michael Monostra for Healio.com/endocrinology, 3 February 2022.
 Optimizing indoor lighting to be brighter during daytime hours and dimmer in the evening may provide cardiometabolic benefits, according to study findings published in Diabetologia. In findings from a randomized controlled trial, participants who were in an indoor environment with bright lighting during the day and dim lighting in the evening had lower plasma glucose levels and increased in energy expenditure compared with bright lighting in the evening and dim lighting during the day, providing evidence that indoor lighting should correspond to the natural day-night cycle.
Optimizing indoor lighting to be brighter during daytime hours and dimmer in the evening may provide cardiometabolic benefits, according to study findings published in Diabetologia. In findings from a randomized controlled trial, participants who were in an indoor environment with bright lighting during the day and dim lighting in the evening had lower plasma glucose levels and increased in energy expenditure compared with bright lighting in the evening and dim lighting during the day, providing evidence that indoor lighting should correspond to the natural day-night cycle.
Read more: Bright indoor lighting during day may lower glucose, improve energy expenditure
FDA must improve medical device interoperability through data standards was reported by Nick Paul Taylor for MedTechDive.com, 31 January 2022. As reported in JAMA, the authors list a range of potential positive outcomes of improved device interoperability, using the experience of the consumer technology and telecommunications industries to make their case.
 While healthcare policy efforts have focused on data sharing between electronic health record (EHR) systems, they have overlooked fragmented data in medical devices, contend researchers, who want to see an agency initiative address the problem head-on.
While healthcare policy efforts have focused on data sharing between electronic health record (EHR) systems, they have overlooked fragmented data in medical devices, contend researchers, who want to see an agency initiative address the problem head-on.
FDA has made the case that medical device interoperability, the ability to safely, securely and effectively exchange and use information among one or more devices, is critical to patient care and reducing errors and adverse events. The agency has also argued that standards are important to the development of reliable interoperable devices from different manufacturers which could lead to new models of healthcare.
However, critics say FDA has not gone far enough in pushing for conformity to medical device interoperability standards.
FDA should encourage the development of an initiative to harmonize medical Internet of Things (IoT) devices to drive the interoperability of medtech products, according to several academics. The authors of an opinion piece published earlier this month in JAMA Health Forum envisage effective medical device interoperability improving healthcare by “enhancing productivity with actionable data.”
Researchers from the University of North Carolina School of Medicine, Columbia University and The Johns Hopkins University School of Medicine set out the current situation and why and how it needs improving. “Medical device data, however, are currently fragmented by device type, ranging from consumer health applications to wearables, ambulance-mounted equipment, and both moveable and stationary devices in clinical settings. This fragmentation underscores the need to facilitate seamless interfacing by providing common language, protocols, principles, and ground rules,” the academics wrote.
Read more: FDA must improve medical device interoperability through data standards
Dr. P Got Covid…His Most Surprising Symptom and Advice If You Get Sick was shared by TCOYD.org, 27 January 2022.
Dr. Jeremy Pettus, endocrinologist and T1D, takes us through his recent experience getting COVID, from what he did first after testing positive to how he treated his symptoms and adjusted his diabetes regimen.





I also do not think that FDA has done enough to force interoperability. We are able to have standards for ethernet cable but not diabetes devices. A standards committee (maybe of an organization developed by the manufacturers) to set standards. Yes, the standards should grow and shift. USB has changed many times, so should the interoperability of insulin devices. I mean come on guys, get out the silos. Let’s face it if MedT does not get out of their silo the terrific pump business will die. No one wants that, and if Dexcom will not play ball they will have eventually have issues from a place like Abott.