Let’s look at DIABETES DEVICES!
FDA clears first smartphone app for insulin delivery: Tandem as reported by Nicole Wetsman, for TheVerge.com, 17 February 2022. t:slim X2 insulin pump users can use the app to program doses rather than the pump itself!
 The Food and Drug Administration cleared a smartphone app from Tandem Diabetes Care to program insulin delivery for its t:slim X2 insulin pump. It’s the first phone app for both iOS and Android to able to deliver insulin. Previously, delivery had to be handled through the pump itself.
The Food and Drug Administration cleared a smartphone app from Tandem Diabetes Care to program insulin delivery for its t:slim X2 insulin pump. It’s the first phone app for both iOS and Android to able to deliver insulin. Previously, delivery had to be handled through the pump itself.
With this update, pump users will be able to program or cancel bolus doses of insulin, which are taken at mealtimes and are crucial in keeping blood glucose levels under control. “Giving a meal bolus is now the most common reason a person interacts with their pump, and the ability to do so using a smartphone app offers a convenient and discrete solution,” said John Sheridan, president and CEO of Tandem Diabetes Care.
The change could be a big improvement for people who prefer not to have pumps out in pubic settings or attach them to undergarments like bras.
Read more:
Senseonics glucose monitor approval overshadowed by ‘eye popping’ fall in sales forecast was posted by Nick Paul Taylor for MedTechDive.com, 14 February 2022.
 Senseonics has won FDA approval for its six-month continuous glucose monitoring system. Yet, the long-awaited approval was overshadowed by 2022 revenue guidance that was around half of what analysts expected. The approval gives Senseonics and partner Ascensia Diabetes Care a more convenient CGM implant to pitch as they seek to gain momentum in a market dominated by companies such as Abbott Laboratories and Dexcom, which make wearable devices that are worn for roughly 10 or 14 days depending on the product. Despite setting a second-quarter launch date for the new product, Senseonics expects little sales growth in 2022, with the bottom end of target guidance range only just beating the $13.7 million generated last year.
Senseonics has won FDA approval for its six-month continuous glucose monitoring system. Yet, the long-awaited approval was overshadowed by 2022 revenue guidance that was around half of what analysts expected. The approval gives Senseonics and partner Ascensia Diabetes Care a more convenient CGM implant to pitch as they seek to gain momentum in a market dominated by companies such as Abbott Laboratories and Dexcom, which make wearable devices that are worn for roughly 10 or 14 days depending on the product. Despite setting a second-quarter launch date for the new product, Senseonics expects little sales growth in 2022, with the bottom end of target guidance range only just beating the $13.7 million generated last year.
Senseonics now has its long-sought approval and a label that matches their expectations. However, Senseonics does not expect the product to drive significant sales growth in 2022. The full-year guidance range is $14 million to $18 million, compared to the $13.7 million the company brought in last year. Analysts expected Senseonics to struggle to hit the upper reaches of the $30 million to $40 million it guided in 2020, but they were forecasting it to reach the bottom end of the range. Analysts called the down-revision “eye popping.”
“Some change was expected given COVID delays, though we struggle to understand why the guide assumes zero growth at the low-end, particularly with a brand-new product approved, the pandemic abating vs. 2021 and Ascensia’s partnership only just beginning,” the analysts wrote in a note to investors. “If Ascensia is holding excess Eversense inventory (either OUS or U.S.) and refuses to purchase more until this inventory is burned down, it could partially explain the weaker guide.”
Read more: Senseonics glucose monitor approval overshadowed by ‘eye popping’ fall in sales forecast
In the Pipeline: Sigi Pump System was broadcast by Stacey Simms on DiabetesConnections.com, 15 February 2022.

Located in the heart of the Swiss Medtech Valley, AMF Medical began an Innosuisse-supported project in 2015 to further develop its technology to address unmet needs of patch pump users, followed in 2018 by the actual product development and design of Sigi, the next-generation plug ‘n go… insulin patch pump. This led then to the creation of AMF Medical SA in early 2021, as a separate company from Advanced Microfluidics to which it owes its technological heritage.
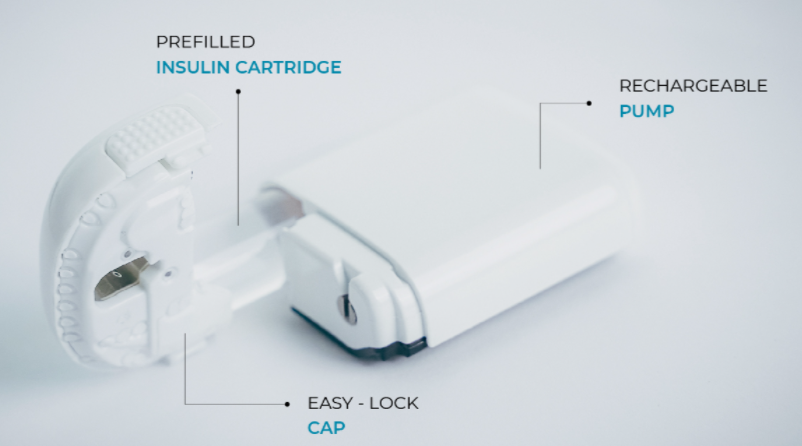
 Sigi can be programmed to deliver basal and bolus insulin at both set and variable rates. In addition, as an Alternate Controller Enabled-pump, it is able to receive, execute and confirm dosing commands by interacting with Bluetooth-compatible, continuous glucose monitors and alternate controller devices, like a smartphone or an iController. Sigi is also smaller, lighter and more convenient than current insulin delivery systems. This is made possible by its ergonomic design, which accommodates standard, insulin pre-filled pump cartridges.
Sigi can be programmed to deliver basal and bolus insulin at both set and variable rates. In addition, as an Alternate Controller Enabled-pump, it is able to receive, execute and confirm dosing commands by interacting with Bluetooth-compatible, continuous glucose monitors and alternate controller devices, like a smartphone or an iController. Sigi is also smaller, lighter and more convenient than current insulin delivery systems. This is made possible by its ergonomic design, which accommodates standard, insulin pre-filled pump cartridges.
Read more: Sigi Insulin Management System
Medtronic talks diabetes group’s FDA warning letter, new products, supply chain constraints was reported by Ricky Zipp for MedTechDive.com, 18 February 2022.
Diabetes technology companies have been some of the most resilient in the medtech sector over the last two years with companies like Dexcom, Insulet, Tandem Diabetes Care and larger Abbott Laboratories who have seen their businesses grow as continuous glucose monitor and insulin pump adoption has taken off. However, amid these success stories, Medtronic has stumbled a bit. CEO Geoff Martha told investors in November that the diabetes group lost market share again the previous quarter, and a recent FDA warning letter has cast uncertainty over the unit, likely delaying key product reviews and subsequently, their launches.
 Ali Dianaty, vice president of product innovation and operations for Medtronic Diabetes, said that the company began working on issues brought up in the agency’s warning letter following an inspection in June and July, roughly six months before Medtronic received the letter. Dianaty said that Medtronic is communicating with the FDA and making necessary changes, but the company still has “a lot of work to do.”
Ali Dianaty, vice president of product innovation and operations for Medtronic Diabetes, said that the company began working on issues brought up in the agency’s warning letter following an inspection in June and July, roughly six months before Medtronic received the letter. Dianaty said that Medtronic is communicating with the FDA and making necessary changes, but the company still has “a lot of work to do.”
The diabetes group is focusing on new products and strategies to keep up with the constantly growing competition in both the CGM and insulin pump markets. New focuses include developing an insulin pump patch product to compete with Insulet and, eventually, Tandem, as well as investing in direct-to-consumer advertising as the company explores delivering products more through the pharmacy channel. “There’s a reason why there’s only one patch provider out there today, Insulet’s Omnipod, because of how difficult it is to make it cost-effective — knowing how much you’re throwing away every three days — and they barely got to a place where they’re profitable. Our internal dilemma has been, how do we make this good for patients and still viable to allow it to grow the right way. Because when you look at total costs for a patch, they’re not small, they’re hard to deal with. That’s what we’ve been spending our time and effort on.”
The semiconductor chip shortage has impacted companies in different ways across the industry. Medtronic went from needing to purchase six months in advance to now between 53 and 85 weeks. It has gotten really, really hard.
Read more: Medtronic talks diabetes group’s FDA warning letter, new products, supply chain constraints
Potential Type 1 Diabetes “Milestone” – the First Trial of Gene-Edited Islet Cells Has Begun was shared by Ross Wollen for DiabetesDaily.com, 16 February 2022.
A truly groundbreaking diabetes trial is now underway: for the first time, a patient has received a transplant of lab-grown insulin-secreting islet cells that have been gene-edited to evade the immune system. The therapy is called VCTX210, and it raises hopes that people with diabetes could one day enjoy recovered insulin production without having to take immunosuppressive drugs. The announcement was made by CRISPR Therapeutics, which developed the innovative gene-editing technique, and ViaCyte, a biotech firm devoted to finding a functional cure for diabetes using stem cell-derived pancreatic cells.
Dr. James Shapiro, the clinical investigator in the new trial is a giant in the field – as a surgeon in the late 1990’s he performed the world’s first islet cell transplants for patients with type 1 diabetes, a technique that was dubbed “the Edmonton protocol.” He is now the director of the Clinical Islet and Liver Transplant Programs at the University of Alberta of Edmonton, Canada.
Pancreatic islet cell transplants have proven to be safe and effective, but they remain rare, partially due to the scarcity of organ donors. ViaCyte has developed a nearly “limitless” supply of pancreatic islet cells – by growing them in a laboratory from pluripotent stem cells. A competitor, Vertex, has also devised a solution using stem cells.
According to Shapiro, any islet cell transplant therapy that requires ongoing immunosuppression will necessarily be limited to a smaller number of patients, mostly those with “really impossible to control type 1 diabetes, patients facing dangerous lows in their blood sugar. And that’s about five percent, perhaps ten percent of the type 1 diabetes population today. And it doesn’t include children.” “Immunosuppressive drugs are the big barrier for why we don’t do large numbers of cell transplants today.” Immunosuppressive drugs can have serious side effects including increased risk of cancers, increased risk of life-threatening infections, side effects on the kidney, and they can also be toxic to the functioning of the transplanted cells and their ability to make insulin.” “So being able to carry out a transplant with no anti-rejection drugs, if it’s successful, would be a milestone advance for cell therapy in this disease.”
Dr. Shapiro explained that pancreatic cell transplants, if they effectively evade the immune system, could be used in a huge number of patients, potentially in “all forms of diabetes.” “If we didn’t have that lifetime risk [from immunosuppressive therapy] ahead of us, we would be able to open the gates and include everybody. Not just adults but children and patients with type 2 diabetes. There’s no reason why this cell replacement therapy wouldn’t work in patients with insulin-requiring type 2 diabetes.”
Gene-editing is not the only proposed method of hiding transplanted islet cells from the immune system. ViaCyte has an alternative solution in the works, a porous pouch that would encapsulate the new islet cells, allowing glucose and insulin to filter across the barrier but barring the larger immune cells. Their competitor Vertex is reportedly working on a similar solution, which they compare to a “teabag.”
 But Dr. Schapiro believes that precision gene-editing with CRISPR could ultimately prove to be the winning strategy. “I think the ability to alter the immune signaling on the cell surface, to make a cell not recognized and not destroyed by the alloimmune system, is going to be a massive advance for all areas of transplantation. Since the 1950s people have been working on the idea of immune tolerance, and the holy grail is transplantation that wouldn’t need any of these immunosuppressive drugs. ViaCyte and CRISPR Therapeutics are really leading the way in that regard.”
But Dr. Schapiro believes that precision gene-editing with CRISPR could ultimately prove to be the winning strategy. “I think the ability to alter the immune signaling on the cell surface, to make a cell not recognized and not destroyed by the alloimmune system, is going to be a massive advance for all areas of transplantation. Since the 1950s people have been working on the idea of immune tolerance, and the holy grail is transplantation that wouldn’t need any of these immunosuppressive drugs. ViaCyte and CRISPR Therapeutics are really leading the way in that regard.”
The new breakthrough trial has begun with its first patient, the first in the world to have received a transplant of these gene-edited islet cells. The patient “tolerated the surgery without missing a beat.” The surgery doesn’t sound terribly invasive, requiring only “tiny little incisions on the abdominal wall.”
“Patients want to hear when it will be available, but they’re also sick of hearing ‘another five years to a cure,’ so we don’t talk about that. We talk about the immediate challenges ahead of us. It would be nice to have a crystal ball, but at the same time, I think the reality is that we work by facing challenges and fixing them. “Maybe these first gene edits will get us a long way there, but maybe they won’t be perfect. I don’t know that yet. Maybe further edits and optimization will be required.”
Would VCTX210, if all goes according to plan, be considered a “cure” for type 1 diabetes?
“We’re always careful about the word ‘cure.’ I think we can say very clearly that this could be far superior to insulin therapy because it provides a potential biological solution to this biological disease. It could provide perfect day-to-day and moment-to-moment control of blood sugar that an injection of insulin from the outside cannot do. Even the closed-loop systems have so much lag when you deliver insulin under the skin, it’s really very inefficient compared to a normal pancreas or islet cell transplants.
“Bottom line: this is an incredibly exciting and important trial. It’s the first-in-human trial, the first patient treated, and now we’re off to the races. For me, it’s been an immense privilege to be part of this, and I am really excited about the potential. There’s a lot happening right now in diabetes, but I think this could be big.”
Read more: Potential Type 1 Diabetes “Milestone” – the First Trial of Gene-Edited Islet Cells Has Begun


It looks like the Medtronic FDA letter is going to have a more profound impact in the US than has recently been discussed. It will be interesting to see how it recovers.