In this week’s issue of The Savvy Diabetic:
-
FDA Petition to Approve Sotagliflozin
-
Dexcom & Oura
-
Late Eating Alters BG Levels
-
PharmaSens NEW Insulin Pumps
-
FDA Approves Medtronic InPen Smart Insulin Pen
-
PBMs sue FTC over Insulin Litigation
YOU CAN MAKE A DIFFERENCE!
Help Impact the FDA’s Decision to Improve Glucose Control in People with Type 1 Diabetes Who are Also Living with Chronic Kidney Disease with an Oral Medication. We call upon the U.S. Food and Drug Administration (FDA) to address the need for new therapeutic options for people with Type 1 diabetes (T1D) who are also living with CKD.
 People with Type 1 Diabetes face significantly higher risks of morbidity and mortality. In the United States, an estimated 1.7 million adults have Type 1 Diabetes, with approximately 21%, or 360,000, also affected by CKD. This petition represents the voices of T1D patients, families, healthcare providers, and advocates who are eager for innovative and effective options to manage this life-altering condition.
People with Type 1 Diabetes face significantly higher risks of morbidity and mortality. In the United States, an estimated 1.7 million adults have Type 1 Diabetes, with approximately 21%, or 360,000, also affected by CKD. This petition represents the voices of T1D patients, families, healthcare providers, and advocates who are eager for innovative and effective options to manage this life-altering condition.
By signing this petition, your voice will be heard and you can help advocate for access to important treatments for the type 1 diabetes community. (No monetary contribution is required to sign this petition and any financial contributions given via change.org do not go to TCOYD.)
If you or a loved one are living with type 1 diabetes then you know the literal blood, sweat, and tears that go into managing this 24-hour 365-days-a-year condition. Did you know that the FDA is considering approving the first oral medication for type 1s living with chronic kidney disease to improve glucose control?
Sotagliflozin is a once daily oral medication that is currently approved by the FDA only in people with type 2 diabetes to improve glycemic control and reduce the risk of a stroke, heart attack, hospitalization, or death from congestive heart failure. Importantly, it is used in people with type 2 diabetes to reduce the progression of chronic kidney disease.
If approved, sotagliflozin would be the first oral medication approved for those living with type 1 diabetes since the discovery of insulin over 100 years ago.
SIGN HERE!
Smart ring maker Oura picks up $75M series D, inks strategic partnership with Dexcom by Heather Landi for FierceHealthcare.com, 19 November 2024.
 Oura is partnering with medical device maker Dexcom to integrate data from glucose biosensors with the Oura Ring, which tracks sleep, heart rate and activity. Oura also picked up $75 million from Dexcom in a series D funding round, valuing the company at more than $5 billion, the company announced Tuesday.
Oura is partnering with medical device maker Dexcom to integrate data from glucose biosensors with the Oura Ring, which tracks sleep, heart rate and activity. Oura also picked up $75 million from Dexcom in a series D funding round, valuing the company at more than $5 billion, the company announced Tuesday.
The company, which launched out of Finland as a sleep tracking smart ring in 2015, has now sold 2.5 million rings. It expects to see annual sales double in 2024 to roughly $500 million, and the company is profitable, according to Oura CEO Tom Hale. The two companies will launch integrations enabling data to flow between Dexcom products, including glucose biosensors and apps, and the Oura Ring and its app, the companies said. The first app integration resulting from the partnership is expected to launch in the first half of 2025.
Read more: Smart ring maker Oura inks strategic partnership with Dexcom
Eating more than 45% of calorie intake after 5 p.m. alters glucose levels by Teresa Bau & Sonia Armengou for MedicalXPress.com, 19 November 2024.
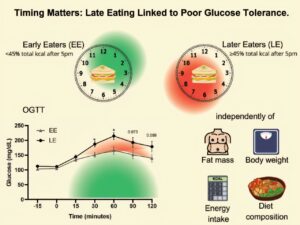 Although people have always said that having a light and early dinner is better, a study by the Universitat Oberta de Catalunya (UOC) and Columbia University has provided the scientific grounds for this argument. According to a study published in Nutrition & Diabetes, consuming more than 45% of our daily calorie intake after 5 p.m. is associated with an increase in glucose levels, with the harmful consequences that this has for health, regardless of the individual’s weight and body fat. The study was carried out at Columbia University’s Irving Medical Center in New York, and was led by Dr. Diana Díaz Rizzolo, postdoctoral researcher and member of the Faculty of Health Sciences at the UOC.
Although people have always said that having a light and early dinner is better, a study by the Universitat Oberta de Catalunya (UOC) and Columbia University has provided the scientific grounds for this argument. According to a study published in Nutrition & Diabetes, consuming more than 45% of our daily calorie intake after 5 p.m. is associated with an increase in glucose levels, with the harmful consequences that this has for health, regardless of the individual’s weight and body fat. The study was carried out at Columbia University’s Irving Medical Center in New York, and was led by Dr. Diana Díaz Rizzolo, postdoctoral researcher and member of the Faculty of Health Sciences at the UOC.
“Maintaining high levels of glucose over long periods can have implications including a higher risk of progressing to type 2 diabetes, an increase in cardiovascular risk due to the damage that high glucose levels do to blood vessels, and increased chronic inflammation, which aggravates cardiovascular and metabolic damage,” said Díaz Rizzolo.
The importance of the study lies in the fact that it shows that the time of day when meals are eaten can in itself have a negative impact on glucose metabolism, regardless of the amount of calories consumed throughout the day and the individual’s weight and body fat.
Read more: Eating more than 45% of calorie intake after 5 p.m. alters glucose levels
PharmaSens’ All-In-One Tubeless Insulin Pump & CGM by Justin Eastzer for Diabetech.beehiiv.com, 20 November 2024.
A new era of insulin delivery is on board with Niia, an innovative tubeless insulin pump under development by PharmaSens. The Swiss diabetes tech company is working on a lineup of three devices, including the world’s first all-in-one insulin pump and CGM system – a huge simplification for people with diabetes.
 The Niia Essential, PharmaSens’ first insulin pump, is awaiting FDA 510(k) clearance and is designed to replace insulin pens for people who need basal and bolus insulin delivery. Unlike the Omnipod 5 or Tandem t:slim X2, this first pump does not yet integrate with a CGM or have closed-loop capabilities. The pump is tubeless and compact, with a 3mL insulin capacity, allowing for an extended wear time that PharmaSens hopes will last 3-7 days.
The Niia Essential, PharmaSens’ first insulin pump, is awaiting FDA 510(k) clearance and is designed to replace insulin pens for people who need basal and bolus insulin delivery. Unlike the Omnipod 5 or Tandem t:slim X2, this first pump does not yet integrate with a CGM or have closed-loop capabilities. The pump is tubeless and compact, with a 3mL insulin capacity, allowing for an extended wear time that PharmaSens hopes will last 3-7 days.
The Niia pumps are semi-reusable. The electronic cover has a two-year lifespan, and the disposable patches and vial holders are replaced with each new infusion set. While bolus dosing is done directly from the pump, Bluetooth connectivity enables users to adjust settings and create treatment profiles via an app.
PharmaSens plans to expand its lineup with a second pump, the Niia Advanced, which will add external CGM connectivity with sensors like Dexcom or Libre. Its third pump, Niia Signature, promises a groundbreaking all-in-one insulin pump and CGM system. This would mean less room for error and more space in our cabinets and suitcases for anything other than diabetes supplies!
An all-in-one device would also mean users wouldn’t need to worry about the loss of connection when swimming! Bluetooth does not work underwater and this is how our phones and diabetes devices connect.
Read more: PharmaSens’ All-In-One Tubeless Insulin Pump & CGM
Medtronic nets FDA nod for smart insulin pen app by Susan Kelly for MedTechDive.com, 22 November 2024.
 Medtronic received Food and Drug Administration clearance for its new InPen smart insulin pen app that can recommend corrections for missed or inaccurate insulin doses at mealtime.
Medtronic received Food and Drug Administration clearance for its new InPen smart insulin pen app that can recommend corrections for missed or inaccurate insulin doses at mealtime.
InPen is a reusable insulin pen that can send dosing information to an app for users. The smart insulin pen also offers dose calculations and tracking for diabetes management. The InPen app calculates insulin doses based on glucose levels and carbohydrate estimates. The new InPen app can alert users of a missed insulin dosage to help minimize the frequency of glucose highs. Simplera is Medtronic’s first disposable CGM and is half the size of the company’s previous CGMs. Medtronic announced FDA approval of the Simplera CGM in August. The company will combine the InPen with its Simplera CGM into the Smart MDI system.
Read more: Medtronic nets FDA nod for smart insulin pen app
UnitedHealth, Cigna, CVS sue FTC over insulin litigation process by Rebecca Pifer for HealthCareDive.com, 20 November 2024.
 CVS, Cigna, and UnitedHealth are suing the Federal Trade Commission to block the agency’s lawsuit against their pharmacy benefit managers for allegedly inflating U.S. insulin prices. The three companies are claiming the FTC’s suit is unconstitutional because it was lodged in the agency’s administrative court instead of a federal court, violating their right to due process under the Fifth Amendment.
CVS, Cigna, and UnitedHealth are suing the Federal Trade Commission to block the agency’s lawsuit against their pharmacy benefit managers for allegedly inflating U.S. insulin prices. The three companies are claiming the FTC’s suit is unconstitutional because it was lodged in the agency’s administrative court instead of a federal court, violating their right to due process under the Fifth Amendment.
The FTC slammed the move as a tactic to distract from the allegations against the PBMs. The FTC’s claims involve private rights, so they must be litigated in federal court, rather than the “fundamentally unfair” in-house process, according to the complaint. In addition, the FTC commissioners, along with their in-house tribunal, are “unconstitutionally insulated from removal by the President and thus are insulated from democratic accountability,” the complaint reads.
“It has become fashionable for corporate giants to argue that a 110-year-old federal agency is unconstitutional to distract from business practices that we allege, in the case of PBMs, harm sick patients by forcing them to pay huge sums for life-saving medicine. It will not work,” FTC spokesperson Douglas Farrar said
Read more: UnitedHealth, Cigna, CVS sue FTC over insulin litigation process

