In this week’s issue of The Savvy Diabetic:
-
New Insulin to Prevent Sudden BG Drops
-
Luna Diabetes Clinical Trial for AID Closed-Loop for MDI
-
Levicure’s Triple-Combo Oral Therapy to Restore Insulin Production
-
GLP-1, SGLT2 Drug Scripts on the Rise for T1Ds
-
Diabetes Technology Society Showcased Innovations in Diabetes Management
-
Semaglutide Linked to Reduced Risk for Alzheimer’s in Diabetics
-
Scientists Track Age You’re Most Likely to be Hospitalized
-
Medicare Advantage Insurers Under Fire by US Senate
-
MultiPlan Accused of Conspiring with Health Insurers to Underpay Doctors
New type of insulin that switches on and off could help diabetics avoid sudden drops in blood sugar levels by Nature Publishing Group for MedicalXpress.com, 16 October 2024.
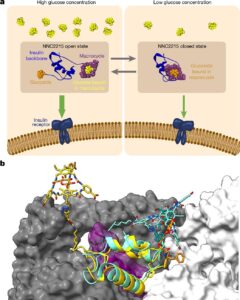 A paper in Nature reports that a modified insulin that can prevent sudden drops in blood sugar has been demonstrated in laboratory experiments and animal models. This could provide a more flexible way to supplement insulin for individuals with diabetes and reduce sudden drops in blood glucose.
A paper in Nature reports that a modified insulin that can prevent sudden drops in blood sugar has been demonstrated in laboratory experiments and animal models. This could provide a more flexible way to supplement insulin for individuals with diabetes and reduce sudden drops in blood glucose.
Researcher Rita Slaaby and colleagues present a modified form of insulin with activity that varies according to glucose levels in the blood. The molecule, NNC2215, is equipped with a switch that can open and close in response to glucose. Under high glucose concentrations, the switch opens, and the insulin becomes more active, removing glucose from the blood. When glucose levels decrease, the switch moves to a closed state, which prevents glucose uptake.
This modified insulin shows promise in preventing the sharp drops in glucose that can severely affect individuals with diabetes, especially during sleep. This could improve both long-term and short-term complications associated with diabetes.
Read more:
Luna Diabetes trials automated wearable insulin pump by GlobalData.com, 24 October 2024.
 US-based device company Luna Diabetes has initiated a pivotal trial evaluating its automated closed-loop insulin technology, the Luna System, in patients with type 1 diabetes (T1D) who require multiple daily insulin injections. The Luna system was developed as a wearable insulin pump and alternative to insulin pen to automate the insulin delivery process. The device works with continuous glucose monitors (CGM) to calculate and deliver rapid-acting insulin doses during sleep.
US-based device company Luna Diabetes has initiated a pivotal trial evaluating its automated closed-loop insulin technology, the Luna System, in patients with type 1 diabetes (T1D) who require multiple daily insulin injections. The Luna system was developed as a wearable insulin pump and alternative to insulin pen to automate the insulin delivery process. The device works with continuous glucose monitors (CGM) to calculate and deliver rapid-acting insulin doses during sleep.
The AID4MDI study will use Time In Range (TIR) as the primary endpoint to assess the system’s effectiveness in maintaining optimal glucose levels. Luna’s pioneering solution integrates the world’s smallest patch pump and a fully closed-loop algorithm, offering a unique approach to improving safety and convenience for individuals with diabetes who cannot or will not use a traditional insulin pump. The study is expected to be completed in early 2025, with a planned commercial launch soon after.
Read more:
Can This Triple-Combo Oral Therapy Restore Insulin Production? by Ginger Vieira for DiabetesDaily.com, 24 October 2024.
 A small biotechnology company in Israel says it can dramatically restore insulin production in people with type 1 diabetes by combining two existing medications with a proprietary version of a common supplement. Levicure’s triple-combo oral therapy is already considered very safe, because it involves two drugs approved by the U.S. Food and Drug Administration (FDA) and a well-known supplement.
A small biotechnology company in Israel says it can dramatically restore insulin production in people with type 1 diabetes by combining two existing medications with a proprietary version of a common supplement. Levicure’s triple-combo oral therapy is already considered very safe, because it involves two drugs approved by the U.S. Food and Drug Administration (FDA) and a well-known supplement.
The therapy is a combination of:
-
-
-
- DPP4-inhibitors, a common type 2 diabetes medication
- Proton pump inhibitors (PPIs), a drug for severe acid reflux
- A proprietary version of gamma-aminobutyric acid (GABA), a supplement often used to treat anxiety
-
-
None of these medications can restore insulin production in a person with type 1 diabetes on their own. But when combined, Levicure says, the triple-combo therapy works to reverse the progression of type 1 diabetes and restore insulin production by targeting issues within both the immune system and the metabolic system. Levicure says the combined effect can block beta cell destruction, suppress autoimmunity, and restore beta cell function.
So far, Levicure’s triple therapy has gone through only one retrospective chart review; it has not been put to the test in a randomized controlled trial, the gold standard for evaluating new drug treatments. Nevertheless, Levicure is excited about the early results, which suggest that the therapy: stops the immune system from attacking beta cells; creates new beta cells; helps damaged beta cells regenerate; preserves existing beta cells; increases insulin production; increases GLP-1 hormone production; reduces how much glucose the liver produces. The results were published in 2023.
Read more:
Scripts for GLP-1, SGLT2 Drugs on the Rise in Type 1 Diabetes Patients by Kristen Monaco for MedPageToday.com, 23 October 2024.
 GLP-1 receptor agonist or SGLT2 inhibitor prescribing became more common for people with type 1 diabetes in recent years, a nationwide cross-sectional analysis found. The FDA has not approved any SGLT2 inhibitor or GLP-1 receptor agonist for type 1 diabetes, but their use in this patient population “may continue due to the significant weight management and cardiorenal benefits observed in people with and without type 2 diabetes.” In their study, the researchers noted that the differences in characteristics for patients prescribed these drug suggest their use “was intended to address additional medical needs.”
GLP-1 receptor agonist or SGLT2 inhibitor prescribing became more common for people with type 1 diabetes in recent years, a nationwide cross-sectional analysis found. The FDA has not approved any SGLT2 inhibitor or GLP-1 receptor agonist for type 1 diabetes, but their use in this patient population “may continue due to the significant weight management and cardiorenal benefits observed in people with and without type 2 diabetes.” In their study, the researchers noted that the differences in characteristics for patients prescribed these drug suggest their use “was intended to address additional medical needs.”
From 2010 to 2023, the percentage of type 1 diabetes patients prescribed an agent from one of these two classes increased from 0.7% to 8.3%, with the bulk of the increase driven by GLP-1 agonists, reported Hui Shao, MD, PhD, of Emory University Rollins School of Public Health in Atlanta, and colleagues. The FDA has not approved any SGLT2 inhibitor or GLP-1 receptor agonist for type 1 diabetes, but their use in this patient population “may continue due to the significant weight management and cardiorenal benefits observed in people with and without type 2 diabetes,” they wrote in JAMA.
Read more: Scripts for GLP-1, SGLT2 Drugs on the Rise in Type 1 Diabetes Patients
Groundbreaking Innovations in Diabetes Care: Highlights from the 2024 Diabetes Technology Meeting by Daniel Trecroci for BeyondType1.org, October 2024.
 The Diabetes Technology Society’s annual Diabetes Technology Meeting was held in Burlingame, California, between October 15th and 17th, 2024. The event showcased groundbreaking innovations in diabetes care, featuring scientists and industry leaders who gathered to present the latest advancements in continuous glucose monitors (CGMs), automated insulin delivery (AID) systems, and artificial intelligence (AI). This year’s event highlighted how emerging technologies are revolutionizing diabetes management and offering greater convenience, accuracy, and personalized care. From cutting-edge research to real-world applications, the meeting underscored the vital role of technology in improving outcomes for people living with diabetes. Here’s a look at some of the exciting developments revealed during the conference.
The Diabetes Technology Society’s annual Diabetes Technology Meeting was held in Burlingame, California, between October 15th and 17th, 2024. The event showcased groundbreaking innovations in diabetes care, featuring scientists and industry leaders who gathered to present the latest advancements in continuous glucose monitors (CGMs), automated insulin delivery (AID) systems, and artificial intelligence (AI). This year’s event highlighted how emerging technologies are revolutionizing diabetes management and offering greater convenience, accuracy, and personalized care. From cutting-edge research to real-world applications, the meeting underscored the vital role of technology in improving outcomes for people living with diabetes. Here’s a look at some of the exciting developments revealed during the conference.
-
-
-
- One Health Biosensing shared its next-generation intradermal CGM device. Their reusable, needle-free sensors are designed for affordability and ease, making CGM accessible for more people with type 2 diabetes. www.onehealthbiosensing.com.
- GlucoCopilot.ai developed an AI-driven platform that analyzes data from CGMs, insulin pumps, and lifestyle factors to provide personalized insights and actionable recommendations by using machine learning. (no available website)
- Perspirion Diagnostics showcased its innovative sweat-based glucose monitoring system which analyzes glucose levels through perspiration, as a non-invasive alternative. www.perspiriontech.com.
- Daia Health is a diabetes management app designed to improve safety for people with type 1 diabetes. It allows users to share real-time blood sugar information with emergency contacts during critical low blood sugar events. www.diai.health.
- Therapon Health offers advanced technology and integration with Electronic Health Records (EHR). www.theraponhealth.com.
- Gluroo is a free, collaborative app designed to simplify diabetes management through real-time data sharing and integration with devices like CGMs, watches, and health apps. Gluroo’s unique features enable families, caregivers, and healthcare providers to monitor and support diabetes management in real-time. www.gluroo.com.
- Zimmer & Peacock has developed and manufactured electrochemical sensors and biosensors for medical diagnostics, wearable technologies, and more. They specialize in functionalizing electrodes with enzymes, RNA, DNA, and other components to create specific sensors, including for CGMs. www.zimmerpeacock.com.
- BOYDSense offers a non-invasive glucose monitoring technology that uses breath analysis instead of blood. www.boydsense.com.
- Lumosfit is focused on decreasing the risk of diabetes through lifestyle modification and personalized health insights, by utilizing digital health technology to tailor exercise, nutrition, and wellness plans. www.lumosfit.com.
- Provigate is developing blood glucose monitoring devices that integrate non-invasive monitoring solutions with wearable devices, allowing real-time glucose tracking without the need for finger pricks. www.provigate.com/en/.
- Digital Hospital Inc. introduced its revolutionary virtual diabetes care platform, designed to offer 24/7 access to healthcare professionals. www.digitalhospitalinc.com.
-
-
Read more: Highlights from the 2024 Diabetes Technology Meeting
Semaglutide linked to reduced risk for Alzheimer’s in patients with diabetes by Robert Herpen for Healio.com/neurology, 24 October 2024.
 In patients with type 2 diabetes, semaglutide was associated with a significantly lower risk for an Alzheimer’s disease diagnosis within 3 years compared with other diabetes medications, according to a study published in Alzheimer’s & Dementia.
In patients with type 2 diabetes, semaglutide was associated with a significantly lower risk for an Alzheimer’s disease diagnosis within 3 years compared with other diabetes medications, according to a study published in Alzheimer’s & Dementia.
“Semaglutide, a glucagon-like peptide-1 receptor agonist, was approved by the FDA for type 2 diabetes mellitus in 2017 and for weight loss in 2021. Both are significant modifiable risk factors for AD,” Rong Xu, PhD, professor of biomedical informatics and director of the Center for AI in Drug Discovery at Case Western Reserve School of Medicine, and colleagues wrote. “Furthermore, semaglutide has demonstrated benefits in managing various other health conditions such as cardiovascular factors, alcohol use, smoking and depression, many of which are also linked to AD risk.”
Building on preclinical evidence that suggests semaglutide (Novo Nordisk) protects against neurodegeneration and neuroinflammation consistent with AD, Xu and colleagues assessed its potential for delaying or preventing AD in a real-world setting.
Read more: Semaglutide linked to reduced risk for Alzheimer’s in patients with diabetes
Scientists tracked the age you’re most likely to be hospitalized by Reda Wigle for NYPost.com, 23 October 2024.
 The Centers for Disease Control and Prevention released data revealing the age range of Americans who visit healthcare centers most — and the results are surprising. According to the report, in 2022, U.S. adults visited healthcare centers an estimated 89.5 million times, or 349.4 visits per 1,000 adults. That represents a hefty uptick in treatment trends, as 82.3 million visits were reported in the previous year. A staggering two-thirds of healthcare visits were disease-related, including digestive conditions and diabetes. Regarding age, the highest visitation rate was found in those aged 55 to 64. These middle-aged folks visited clinics at a rate of 418 per 1,000 people, compared to 325 in adults from 18 to 44 and 315 in those over 65.
The Centers for Disease Control and Prevention released data revealing the age range of Americans who visit healthcare centers most — and the results are surprising. According to the report, in 2022, U.S. adults visited healthcare centers an estimated 89.5 million times, or 349.4 visits per 1,000 adults. That represents a hefty uptick in treatment trends, as 82.3 million visits were reported in the previous year. A staggering two-thirds of healthcare visits were disease-related, including digestive conditions and diabetes. Regarding age, the highest visitation rate was found in those aged 55 to 64. These middle-aged folks visited clinics at a rate of 418 per 1,000 people, compared to 325 in adults from 18 to 44 and 315 in those over 65.
According to the CDC, the former demographic may have higher visits than others because the majority of new diabetes diagnoses are in people 45 to 64 years old, and the highest rate of reason for visitation, as previously mentioned, was for diseases including diabetes.
Read more: Scientists tracked the age you’re most likely to be hospitalized
Insurance Companies and their Woes
- Senate report slams Medicare Advantage insurers for using predictive technology to deny claims by Susanna Vogel for HealthCareDive.com, 21 October 2024. UnitedHealth, CVS and Humana used technology to increase MA prior authorization denials for post-acute services, boosting profits
 A new Senate report sharply criticizes the country’s three largest Medicare Advantage insurers — UnitedHealthcare, Humana and CVS — for allegedly limiting access to post-acute care to maximize profits. The insurers leveraged algorithmic tools to sharply increase claims denials for MA beneficiaries between 2019 and 2022, according to the report published Thursday by the Senate Permanent Subcommittee on Investigations. They most often denied coverage to patients in nursing homes, inpatient rehab hospitals and long-term hospitals.
A new Senate report sharply criticizes the country’s three largest Medicare Advantage insurers — UnitedHealthcare, Humana and CVS — for allegedly limiting access to post-acute care to maximize profits. The insurers leveraged algorithmic tools to sharply increase claims denials for MA beneficiaries between 2019 and 2022, according to the report published Thursday by the Senate Permanent Subcommittee on Investigations. They most often denied coverage to patients in nursing homes, inpatient rehab hospitals and long-term hospitals.
MA payers have previously come under fire from lawmakers for using algorithms to determine coverage. UnitedHealth and Humana have also been sued for denying MA beneficiaries care using the technology.
Read more: Senate report slams Medicare Advantage insurers for using predictive technology to deny claims
**************************************************************
- AMA sues MultiPlan, insurers alleging ‘cartel’ to fix physician prices by Rebecca Pifer for HealthCareDive.com, 25 October 2024. MultiPlan, which denies the allegations, has been sued dozens of times over concerns the company is conspiring with health insurers to underpay doctors for out-of-network care.
 Cost management company MultiPlan is facing yet another lawsuit for allegedly conspiring to underpay providers — this time, from the largest physician association in the United States.
Cost management company MultiPlan is facing yet another lawsuit for allegedly conspiring to underpay providers — this time, from the largest physician association in the United States.
The American Medical Association’s complaint, filed in an Illinois district court, accuses MultiPlan of colluding with major health insurers to set artificially low reimbursement rates for out-of-network care, forcing providers to accept payments that often don’t cover their operating costs. The litigation, which asks the judge for an injunction requiring MultiPlan to halt the illegal practices, is the latest in a long string of suits against the company. Congress is also scrutinizing MultiPlan, which denies the allegations.
Read more: AMA sues MultiPlan, insurers alleging ‘cartel’ to fix physician prices

