Insulet wins preliminary injunction against EOFlow in patent suit by Susan Kelly for MedTechDive.com, 9 October 2023. A federal judge has blocked EOFlow, a company that Medtronic plans to acquire, from selling its insulin patch pump.
 The U.S. District Court for the District of Massachusetts issued a preliminary injunction preventing EOFlow from selling any product developed using trade secrets from Insulet, according to court documents. Insulet sued EOFlow, the maker of a wearable insulin patch pump that is on track to become part of Medtronic, in August. It accused EOFlow of violating three patents for its Omnipod patch pump. The legal setback for EOFlow comes as Medtronic is preparing to pay $738 million to buy the company for its EOPatch system.
The U.S. District Court for the District of Massachusetts issued a preliminary injunction preventing EOFlow from selling any product developed using trade secrets from Insulet, according to court documents. Insulet sued EOFlow, the maker of a wearable insulin patch pump that is on track to become part of Medtronic, in August. It accused EOFlow of violating three patents for its Omnipod patch pump. The legal setback for EOFlow comes as Medtronic is preparing to pay $738 million to buy the company for its EOPatch system.
EOFlow’s technology is authorized in Europe, South Korea, Indonesia, and the United Arab Emirates but is not yet available in the U.S. The tubeless, wearable, and disposable device is paired with a smartphone application that allows users to control the patch from their phone. This preliminary injunction issued by U.S. District Court Chief Judge F. Dennis Saylor restrains EOFlow from manufacturing, marketing, or selling any product designed using Insulet trade secrets pending a trial on the merits of the case.
Read more: Insulet wins preliminary injunction against EOFlow in patent suit
Most adults with diabetes report emotional distress, but do not discuss it with care team by Michael Monostra for Healio.com/endocrinology, 11 October 2023.
 The majority of adults with diabetes say their diabetes team has never discussed the topic of mental health with them, even though most self-reported feelings of emotional distress, according to survey data published in Diabetic Medicine.
The majority of adults with diabetes say their diabetes team has never discussed the topic of mental health with them, even though most self-reported feelings of emotional distress, according to survey data published in Diabetic Medicine.
“This report confirms current literature about the high prevalence of emotional distress amongst people living with diabetes,” Katharine Barnard-Kelly, PhD, chief science officer at Spotlight Consultations Ltd. in Portsmouth, U.K., and professor at the Southern Health NHS Foundation Trust, and colleagues wrote. “In this large participant sample, the most commonly endorsed experiences were self-reported anxiety and depression as well as fear of low blood sugars, low mood, and diabetes-related distress.”
I’m curious. Does your care team ask about your levels of emotional distress? If they do, do they offer help? Do you feel they are empathetic?
Read more: Most adults with diabetes report emotional distress, but do not discuss it with care team
Best Buy to sell continuous glucose monitoring systems by Rebecca Pifer for MedTechDive.com, 9 October 2023.Best B
 Best Buy plans to start selling continuous glucose monitors in the next few weeks, in the tech retailer’s first foray into prescription-based medical device sales. The company plans to sell the Dexcom G7 CGM at launch and is looking to offer additional CGM systems from other manufacturers, according to the company.
Best Buy plans to start selling continuous glucose monitors in the next few weeks, in the tech retailer’s first foray into prescription-based medical device sales. The company plans to sell the Dexcom G7 CGM at launch and is looking to offer additional CGM systems from other manufacturers, according to the company.
Customers who want to buy a CGM will be routed to the virtual care platform Wheel, where clinicians will determine a patient’s eligibility and write a prescription. Pharmacy tech provider HealthDyne will receive and process prescriptions and consumers can then purchase the CGMs on Best Buy’s website for delivery to their homes.
At Best Buy, the Dexcom G7 will cost $179.99 for a 30-day supply. Best Buy is not currently accepting insurance, and the retailer did not say whether it plans to do so. Best Buy aims to offer consumers a simplified prescribing experience through Wheel and the convenience of home delivery, according to the company. In addition, one prescription offers monthly refills for a year.
Dexcom G7s are also available for $180 or less without insurance with a GoodRx coupon. CGMs are more affordable with insurance. For example, the Dexcom G7 costs roughly $35 with insurance if purchased at Amazon Pharmacy, which also offers home delivery.
Read more: Best Buy to sell continuous glucose monitoring systems
New Hope for Treating Chronic Kidney Disease in Type 1 Diabetes by Michael Howerton for diaTribe.org, 10 October 2023.
 New research is sorely needed to test therapies for chronic kidney disease for those with type 1 diabetes, such as there is for type 2 diabetes. Researchers at EASD 2023 pointed to a new study, FINE-ONE, which will be recruiting in the coming months and promises to add new data to the quest.
New research is sorely needed to test therapies for chronic kidney disease for those with type 1 diabetes, such as there is for type 2 diabetes. Researchers at EASD 2023 pointed to a new study, FINE-ONE, which will be recruiting in the coming months and promises to add new data to the quest.
Historically, there have been few clinical trials testing medications for CKD in type 1 diabetes. The problem has long been the absence of enough clinical trials testing these drugs in the type 1 diabetes population, which has been considerably harder to study for renal damage and failure. Many drugs approved for type 2 diabetes – including SGLT-2 inhibitors and GLP-1 receptor agonists, as well as finerenone (sold under the name Kerendia) – lower glucose levels and also protect kidney and cardiovascular health.
In addition to the lack of clinical trials, the fear that these medications could dangerously lower glucose levels and result in hypoglycemia, and perhaps even cause euglycemic DKA (EDKA), has hampered research. Recognizing the possibility of hypoglycemia and EDKA, caution is warranted, such as perhaps requiring continuous glucose monitors (CGM) to protect against dangerous lows. Ultimately, researchers maintained that this population deserves trials to explore access to these treatments that hold such potential to improve and save lives.
Dr. Kathleen Tuttle of the University of Washington presented a sophisticated algorithm using electronic health records to better identify patients with type 1 diabetes and CKD. She also emphasized the increased risk of both kidney and heart disease in type 1 diabetes. There is a critical need for more research and large-scale studies, Tuttle said, citing the overall prevalence of CKD in those with type 1 diabetes of nearly 30%.
Furthermore, Tuttle said, kidney complications increase with age, with CKD affecting 38% of those with type 1 over 60 years old, and at more than 70% for those over 80 years. More than half of all people with type 1 diabetes have stage 3 CKD and, disturbingly, more than 1 in 5 overall have kidney failure, likely requiring dialysis or a transplant.
Read more: New Hope for Treating Chronic Kidney Disease in Type 1 Diabetes
Vertex Releases New Data on Potential Cure for Type 1 Diabetes by Anna Brooks and Andrew Briskin for diaTribe.org, 7 October 2023.
 A third person with type 1 diabetes has achieved insulin independence after treatment with VX-880, a novel stem cell-derived beta cell therapy developed by Vertex Pharmaceuticals. A third person with type 1 diabetes has achieved insulin independence after treatment with VX-880, a novel stem cell-derived beta cell therapy developed by Vertex Pharmaceuticals. While Vertex’s clinical trial is still in early phases, the new data supports VX-880 as a potentially revolutionary treatment for people with type 1 diabetes. In addition, all six participants in the trial experienced significant improvements in A1C, time in range, as well as a reduced need for insulin injections.
A third person with type 1 diabetes has achieved insulin independence after treatment with VX-880, a novel stem cell-derived beta cell therapy developed by Vertex Pharmaceuticals. A third person with type 1 diabetes has achieved insulin independence after treatment with VX-880, a novel stem cell-derived beta cell therapy developed by Vertex Pharmaceuticals. While Vertex’s clinical trial is still in early phases, the new data supports VX-880 as a potentially revolutionary treatment for people with type 1 diabetes. In addition, all six participants in the trial experienced significant improvements in A1C, time in range, as well as a reduced need for insulin injections.
The new findings were presented at the 2023 European Association for the Study of Diabetes (EASD) conference in Hamburg, Germany. Before VX-880 treatment, all study participants produced zero insulin and had a history of severe, recurring episodes of hypoglycemia (low blood sugar). Three months after cell transplantation, participants exhibited insulin production that successfully responded to glucose.
“These data are particularly meaningful in the context of our overall investigational type 1 diabetes program,” said Dr. Carmen Bozic, chief medical officer at Vertex, in a press release. “We are moving with urgency to bring these potentially transformative therapies to patients who are waiting.”
Read more: Vertex Releases New Data on Potential Cure for Type 1 Diabetes
FDA clears Biomea’s IND for trial of type 1 diabetes therapy by ClinicalTrialsArena.com, 6 October 2023.
 The US Food and Drug Administration (FDA) has granted clearance to Biomea Fusion’s investigational new drug (IND) application, enabling the initiation of the Phase II COVALENT-112 clinical trial of BMF-219 for the treatment of type 1 diabetes (T1D). BMF-219 has been shown to regenerate and retain insulin-producing beta cells in animal models of type 1 and type 2 diabetes in preclinical studies.
The US Food and Drug Administration (FDA) has granted clearance to Biomea Fusion’s investigational new drug (IND) application, enabling the initiation of the Phase II COVALENT-112 clinical trial of BMF-219 for the treatment of type 1 diabetes (T1D). BMF-219 has been shown to regenerate and retain insulin-producing beta cells in animal models of type 1 and type 2 diabetes in preclinical studies.
“We have now established a clinical development plan that we believe will fully explore the potential of BMF-219 across the spectrum of diabetes. The next quarters will be very exciting, as we expect these studies will begin to read out.”
Read more: FDA clears Biomea’s IND for trial of type 1 diabetes therapy
Scientists unlock the secrets of a sixth basic flavor by University of Southern California for Phys.org, 5 October 2023.
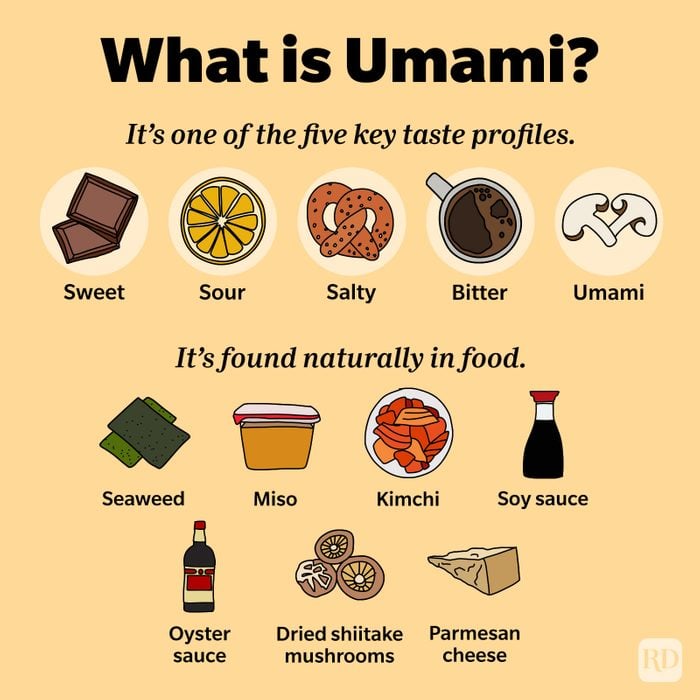 Japanese scientist Kikunae Ikeda first proposed umami as a basic taste—in addition to sweet, sour, salty, and bitter—in the early 1900s. About eight decades later, the scientific community officially agreed with him. Now, scientists led by researchers at the USC Dornsife College of Letters, Arts and Sciences have evidence of a sixth basic taste. In research published in Nature Communications, USC Dornsife neuroscientist Emily Liman and her team found that the tongue responds to ammonium chloride through the same protein receptor that signals sour taste.
Japanese scientist Kikunae Ikeda first proposed umami as a basic taste—in addition to sweet, sour, salty, and bitter—in the early 1900s. About eight decades later, the scientific community officially agreed with him. Now, scientists led by researchers at the USC Dornsife College of Letters, Arts and Sciences have evidence of a sixth basic taste. In research published in Nature Communications, USC Dornsife neuroscientist Emily Liman and her team found that the tongue responds to ammonium chloride through the same protein receptor that signals sour taste.
“If you live in a Scandinavian country, you will be familiar with and may like this taste,” says Liman, professor of biological sciences. In some northern European countries, salt licorice has been a popular candy at least since the early 20th century. The treat counts among its ingredients salmiak salt, or ammonium chloride.
In recent years, Liman and the research team uncovered the protein responsible for detecting sour taste. That protein, called OTOP1, sits within cell membranes and forms a channel for hydrogen ions moving into the cell. Hydrogen ions are the key component of acids, and as foodies everywhere know, the tongue senses acid as sour.
Read more: Scientists unlock the secrets of a sixth basic flavor
Nurse practitioners sue for right to use “doctor” label by Tanya Albert Henry for AMA-Assn.org, 15 September 2023.
 Three nurse practitioners who earned doctorates of nursing practice are suing the state of California, saying that they have earned the right to tout themselves using the term “doctor.” The nurse practitioners claim a California statute that only allows California-licensed allopathic and osteopathic physicians to use the terms “doctor” and “Dr.” is unconstitutional. The restriction has been on the books since at least 1937 to avoid patient confusion over the level of education their health professionals have achieved.
Three nurse practitioners who earned doctorates of nursing practice are suing the state of California, saying that they have earned the right to tout themselves using the term “doctor.” The nurse practitioners claim a California statute that only allows California-licensed allopathic and osteopathic physicians to use the terms “doctor” and “Dr.” is unconstitutional. The restriction has been on the books since at least 1937 to avoid patient confusion over the level of education their health professionals have achieved.
Physicians disagree, telling the court that the breadth of physician education and practical training eclipses that of nurses’ training and that the law avoids patient confusion over who is treating them. In addition, physicians tell the court that surveys support patients’ need and desire to only allow MDs and DOs to call themselves “doctor” or “Dr.”
AMA President Jesse M. Ehrenfeld, MD, MPH, said that “patients want and deserve clarity and transparency in who is providing their care as there are immense differences in the education, training and qualifications among health care professionals.”
WHAT DO YOU THINK?
Read more: Nurse practitioners sue for right to use “doctor” label
The Average Human Body Temperature Is Not 98.6 Degrees by Dana G. Smith for NYTimes.com, 12 October 2023
 Over the past few decades, evidence has been mounting that the average human body temperature is not really 98.6 degrees Fahrenheit. Instead, most people’s baseline is a little bit cooler. The standard of 98.6 was established over 150 years ago by the German physician Dr. Carl Wunderlich, who reportedly took over a million measurements from 25,000 people. Temperatures ranged from 97.2 to 99.5, and the average was 98.6. Dr. Wunderlich also established 100.4 degrees as “probably febrile.”
Over the past few decades, evidence has been mounting that the average human body temperature is not really 98.6 degrees Fahrenheit. Instead, most people’s baseline is a little bit cooler. The standard of 98.6 was established over 150 years ago by the German physician Dr. Carl Wunderlich, who reportedly took over a million measurements from 25,000 people. Temperatures ranged from 97.2 to 99.5, and the average was 98.6. Dr. Wunderlich also established 100.4 degrees as “probably febrile.”
However, a study published in September that evaluated the temperatures of more than 126,000 people between 2008 and 2017 found that the average is closer to 97.9 degrees. Other modern-day studies have reported similar numbers. Experts who study body temperature have differing opinions about why we appear to have gotten cooler over time, and whether that matters when it comes to evaluating fevers and diagnosing infections.
Read more: The Average Human Body Temperature Is Not 98.6 Degrees


so much news:
I predict EOFlow will never be sold int he US unless they pay INsulet a royalty or they change their product.
I have a Best Buy Card will that get me a discount?
We need to know the number of failures Vertex has had before we get excited. If they are 3 for 3 well I am a believer. But if they are 3 for 100, well that is different.
When My neph mentioned me using T2 medications in the future of CKD, I was like, yeah well we will cross that bridge if we ever get there. I mean that is scary to me for a whole range of reasons.
Looking forward to traveling to CA in December. Sheryl and i will be in Carlsbad for a week in early December.
In other news, I am on my way to being a preacher, did I tell you? Its true.