FDA authorizes first interoperable insulin pump intended to allow patients to customize treatment through their individual diabetes management devices, as announced on the FDA website, 14 February 2019 (happy Valentine’s!).
The U.S. Food and Drug Administration today permitted marketing of the Tandem Diabetes Care t:Slim X2 insulin pump with interoperable technology (interoperable t:Slim X2) for delivering insulin under the skin for children and adults with diabetes. This new type of insulin pump, referred to as an alternate controller enabled (ACE) infusion pump, or ACE insulin pump, is the first interoperable pump, meaning it can be used with different components that make up diabetes therapy systems, allowing patients to tailor their diabetes management to their individual device preferences. Diabetes therapy systems may be comprised of an ACE insulin pump and other compatible medical devices, including automated insulin dosing (AID) systems, continuous glucose monitors (CGMs), blood glucose meters or other electronic devices used for diabetes management.
“Diabetes is a complicated disease that requires close monitoring and carefully tailored treatments. We’ve heard from the patient community that having the ability to customize their own diabetes management devices is important to them. Advances in digital health make more tailored approaches to diabetes care possible,” said FDA Commissioner Scott Gottlieb, M.D.
Read more: FDA authorizes first interoperable insulin pump
Looking for patients with type 1 diabetes to compare new drug MYL-1601D with Novolog was reported on Medivisor.com, 17 February 2019.
 This trial is examining the safety and effectiveness of the new drug MYL-1601D (made by Mylan, makers of Glargine long-acting insulin) in comparison to Novolog (fast-acting insulin) in the treatment of type 1 diabetes (T1D). The main outcomes to be measured are the antibody response (response of the immune system to the drug) and improvements in blood glucose control. This study is being carried out in Texas, the United States.
This trial is examining the safety and effectiveness of the new drug MYL-1601D (made by Mylan, makers of Glargine long-acting insulin) in comparison to Novolog (fast-acting insulin) in the treatment of type 1 diabetes (T1D). The main outcomes to be measured are the antibody response (response of the immune system to the drug) and improvements in blood glucose control. This study is being carried out in Texas, the United States.
Read more: Looking for patients with type 1 diabetes to compare new drug MYL-1601D with Novolog
CGM Accuracy – Calibration is King! was written by David Burren on Bionic Wookiee, 15 November 2018 … and is really a worthwhile read.
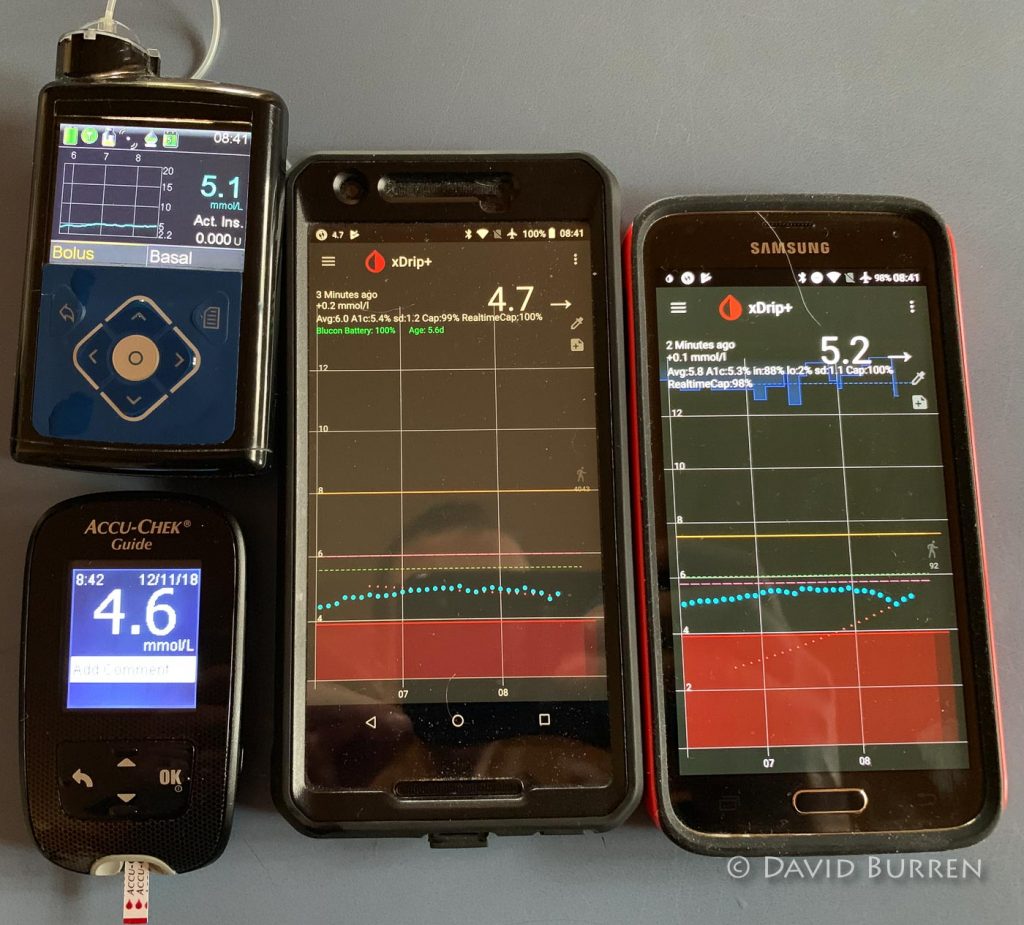 All these CGM systems can be accurate. But only if they’re calibrated well. Poor calibrations will stuff up any system! Unfortunately this is a major “pain point” of current CGM technology.
All these CGM systems can be accurate. But only if they’re calibrated well. Poor calibrations will stuff up any system! Unfortunately this is a major “pain point” of current CGM technology.
But if you keep these concepts in mind you can turn most CGMs into very useful tools.
- Only calibrate when your levels are flat, and likely to remain flat for a while.
Don’t get stuck into a meal immediately after applying a calibration test. - Keep in mind that the systems need more than one calibration point to be able to extrapolate well.
Read more: CGM accuracy – Calibration is King!
By 2025, a lot more people will be tracking their blood sugar, predicts doctor — here’s why, as presented by Aaron Neinstein, M.D., for CNBC Health Tech Matters, 1 February 2019.
 Let’s start with a prediction: By 2025, everyone with diabetes will be tracking their blood sugar with devices called continuous glucose monitors, and it will be common for many people without diabetes to dabble in tracking, too, according to Neinstein.
Let’s start with a prediction: By 2025, everyone with diabetes will be tracking their blood sugar with devices called continuous glucose monitors, and it will be common for many people without diabetes to dabble in tracking, too, according to Neinstein.
- In the future, one doctor suggests that a lot more people without diabetes will dabble in tracking their blood sugar.
- The technology is becoming cheaper and more accessible than ever before, and it will likely get smarter thanks to collaborations between between the device companies and tech companies like Alphabet or Apple.
- Providing consumers with that kind of feedback about their health is powerful.
I TOTALLY AGREE … and will go out on a limb and say that I believe all T1s should be using CGM today … it is the gold standard of care!
Read more: By 2025, a lot more people will be tracking their blood sugar
Companion Medical Announces U.S. Commercial Launch of Smart Insulin Pen System was reported on PRNewsWire.com, 14 December 2018.
 Companion Medical, a leader in the development of advanced technology to improve diabetes care, announced today that it has begun commercial sales of the InPen® system in the United States. InPen is available by prescription only and is a covered benefit under many insurance plans. The InPen is for use with Humalog and Novolog and indicated for persons aged 12 years and older. It comes in 3 colors to help avoid drug confusion. The Companion App home screen gives a summary of your current data. It provides last dose and last blood glucose information, an active insulin display (like an insulin pump) and a graphical depiction of the last 10 hours. In addition, you can access the dose calculator to help with the diffcult calculations required for insulin dosing.
Companion Medical, a leader in the development of advanced technology to improve diabetes care, announced today that it has begun commercial sales of the InPen® system in the United States. InPen is available by prescription only and is a covered benefit under many insurance plans. The InPen is for use with Humalog and Novolog and indicated for persons aged 12 years and older. It comes in 3 colors to help avoid drug confusion. The Companion App home screen gives a summary of your current data. It provides last dose and last blood glucose information, an active insulin display (like an insulin pump) and a graphical depiction of the last 10 hours. In addition, you can access the dose calculator to help with the diffcult calculations required for insulin dosing.
InPen is the first and only FDA-cleared solution that combines an insulin injector pen with an intuitive smartphone app and bolus advisor using Bluetooth® technology. InPen tracks insulin doses, including priming, and automatically sends the data to the user’s mobile device, providing decision support for the constant monitoring and calculating necessary for successful insulin therapy. InPen includes technology to:
- Calculate and recommend optimal dosing;
- Track history and timing of doses for a full year;
- Monitor insulin temperature;
- Remind the user when to take insulin;
- Display last dose and insulin-on-board; and
- Generate actionable reports for the healthcare provider.
Read more: Companion Medical Announces U.S. Commercial Launch of Smart Insulin Pen System
6 Diabetes Research Studies to Watch in 2019 was reported by Julie Workman on ASweetLife.org, February 2019. Here’s a rundown of some research to keep an eye on this year.

- Joslin is currently working on research to determine whether or not naturally occurring betaine supplements (found in grains, beets and other vegetables) have the capability to reduce the risk of developing diabetes in people with prediabetes.
- JDRF has provided funding to Pandion Therapeutics in order to launch trials for autoimmune and inflammatory issues specifically related to type 1 diabetes. Pandion Therapeutics, a biotechnology company out of Cambridge, MA, will aim to develop tethers that are islet-specific and can be paired with certain immunomodulators previously developed by Pandion.
- A fairly new player on the scene, Diabetes Research Connection is a non-profit organization that has been funding diabetes research projects since 2015. One project focuses on hyaluronan (HA), a tissue component that is present during inflammation, is particularly elevated during the early stages of T1D, and tends to cause damage to insulin-producing cells. Researchers aim to study non-diabetic pancreatic tissue in order to understand the timing and causality of these HA cell abnormalities, with the goal of finding a way to stop them.
- Focusing on problems associated with inflammation in T1D, the POSEIDON study includes participants aged between 6 and 65 years old who have been diagnosed with type 1 diabetes within the past ten years. The research in this study will compare the effectiveness of Vitamin D and Omega-3 medications to determine if they are helpful in slowing or stopping the progression of the autoimmune issues in T1D.
- ViaCyte announced that a clinical trial has begun in Europe in 2019, allowing T1D patents to receive a subtherapeutic dose of a human stem cell-derived product. PEC-Direct is an encapsulated “pancreatic progenitor cell product” candidate designed as a beta cell replacement. The device, implanted into the body during a minor surgical procedure, is about the size of a credit card.
- A collaboration between Eli Lilly and Sigilon Therapeutics has been partially funded by JDRF and is expected to continue in pre-clinical stages in 2019. The concept is that insulin-producing islet cells (contained in capsules) would be placed into the abdomen of a person with T1D, lasting for a year without the need for immunosuppressant medicines. The elimination of the body’s immune system response could change everything for this type of cell replacement treatment.
Read more: 6 Diabetes Research Studies to Watch in 2019


The partnership of Eli Lilly and Sigilon Therapeutics has the potential to be wonderful or a totally of no consequence. I do love make and breaks.
Fun to watch … getting a little impatient though!