
Mar 31, 2025 | Advocacy, Diabetes Business, Featured, Research News, Technology Updates
In this week’s issue of The Savvy Diabetic: Dexcom’s FDA Warning Letter & Dexcom’s Response Claims No Wrongdoing CGM MARD Wars, with Values without Context Looking for Glucose Patterns – See Trends & Possible Adjustments...

Mar 24, 2025 | Advocacy, Diabetes Business, Exercise, Featured, Research News, Technology Updates
In this week’s issue of The Savvy Diabetic: Breakthrough T1D Gratitude for Extension of Special Diabetes Program Funding Cut to Diabetes Prevention Program (DPP) Sequel Partners Integrates with Abbott FreeStyle Libre 3 Plus with Twiist Pump Afrezza Lowers...
Mar 17, 2025 | Diabetes Business, Featured, Research News, Technology Updates
In this week’s issue of The Savvy Diabetic: Sync Your CMG to Any Smartwatch, by Diabetotech.com New Biosensor from Bacterial Spores for Glucose Monitoring New iCan CGM from Sinocare in EU New Antibody Test for Celiac Disease for T1D Environmental Waste...
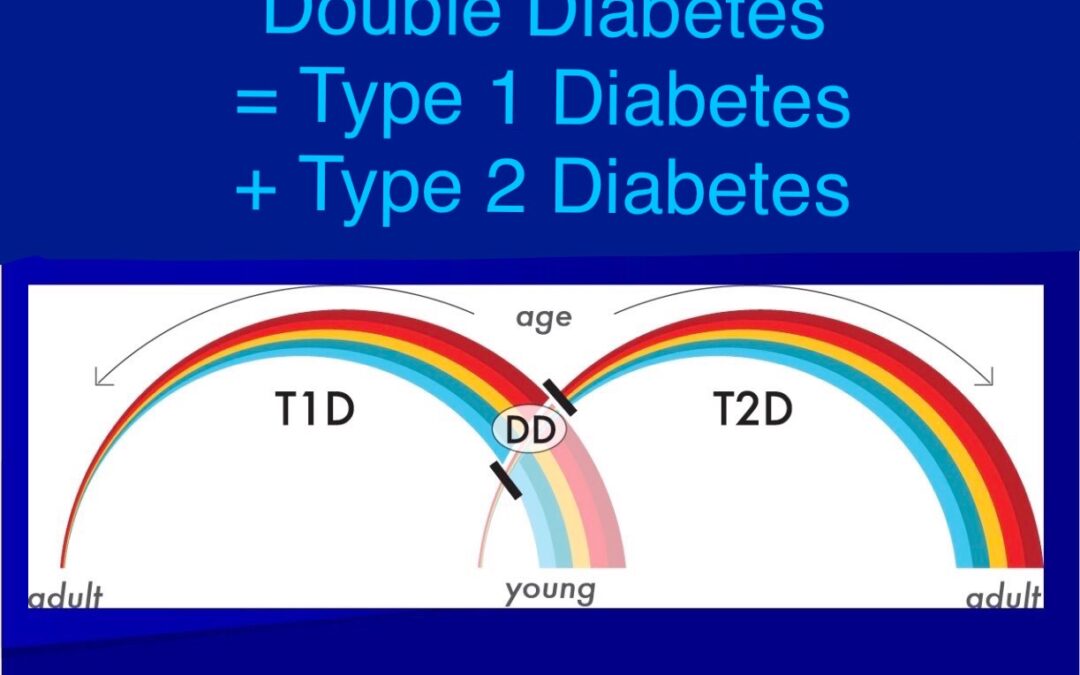
Mar 10, 2025 | Advocacy, Diabetes Business, Featured, Research News, Technology Updates, Videos
In this week’s issue of The Savvy Diabetic: Dexcom’s FTC Warning Letter over Deficiencies Double Diabetes: Silent Threat Hiding in Type 1 Patients Mounjaro vs. Ozempic: Which One Is Right for You? Sleep Duration & Timing and Impact on...

Mar 3, 2025 | A Little Humor, Diabetes Business, Eating, Featured, Foods, Research News, Technology Updates
In this week’s issue of The Savvy Diabetic: Dexcom G7 Shortage (video by Dr. David Ahn) Dexcom G6 Receiver Recall Tandem Control-IQ+ for T2Ds R-VECs for Survival of Insulin-Producing Cells Kadimastem and iTolerance Cell Therapies for Neuro Diseases &...

Feb 24, 2025 | Diabetes Business, Featured, Research News, Stories of D Life, Technology Updates
In this week’s issue of The Savvy Diabetic: Aspartame Triggers Insulin Spike Gut bacteria Alters Brain Proteins Rising World Diabetes Prevalence Medtronic Pump Air Travel Warning FTC Case Against PBMs Move Forward Happy Doctors = Practice Autonomy Sage...








